Iron limitation promotes the atrophy of skeletal myocytes, whereas iron supplementation prevents this process in the hypoxic conditions
- PMID: 29436580
- PMCID: PMC5846664
- DOI: 10.3892/ijmm.2018.3481
Iron limitation promotes the atrophy of skeletal myocytes, whereas iron supplementation prevents this process in the hypoxic conditions
Abstract
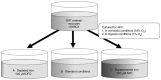
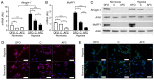
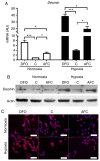
There is clinical evidence that patients with heart failure and concomitant iron deficiency have increased skeletal muscle fatigability and impaired exercise tolerance. It was expected that a skeletal muscle cell line subjected to different degrees of iron availability and/or concomitant hypoxia would demonstrate changes in cell morphology and in the expression of atrophy markers. L6G8C5 rat skeletal myocytes were cultured in normoxia or hypoxia at optimal, reduced or increased iron concentrations. Experiments were performed to evaluate the iron content in cells, cell morphology, and the expression of muscle specific atrophy markers [Atrogin1 and muscle‑specific RING‑finger 1 (MuRF1)], a gene associated with the atrophy/hypertrophy balance [mothers against decapentaplegic homolog 4 (SMAD4)] and a muscle class‑III intermediate filament protein (Desmin) at the mRNA and protein level. Hypoxic treatment caused, as compared to normoxic conditions, an increase in the expression of Atrogin‑1 (P<0.001). Iron‑deficient cells exhibited morphological abnormalities and demonstrated a significant increase in the expression of Atrogin‑1 (P<0.05) and MuRF1 (P<0.05) both in normoxia and hypoxia, which indicated activation of the ubiquitin proteasome pathway associated with protein degradation during muscle atrophy. Depleted iron in cell culture combined with hypoxia also induced a decrease in SMAD4 expression (P<0.001) suggesting modifications leading to atrophy. In contrast, cells cultured in a medium enriched with iron during hypoxia exhibited inverse changes in the expression of atrophy markers (both P<0.05). Desmin was upregulated in cells subjected to both iron depletion and iron excess in normoxia and hypoxia (all P<0.05), but the greatest augmentation of mRNA expression occurred when iron depletion was combined with hypoxia. Notably, in hypoxia, an increased expression of Atrogin‑1 and MuRF1 was associated with an increased expression of transferrin receptor 1, reflecting intracellular iron demand (R=0.76, P<0.01; R=0.86, P<0.01). Hypoxia and iron deficiency when combined exhibited the most detrimental impact on skeletal myocytes, especially in the context of muscle atrophy markers. Conversely, iron supplementation in in vitro conditions acted in a protective manner on these cells.
Conflict of interest statement
Wrocław Medical University received an unrestricted grant from Vifor Pharma outside the submitted work. Monika Kasztura reports financial support from Vifor Pharma for travel and accommodation for scientific meeting. Waldemar Banasiak reports personal fees from Vifor Pharma, outside the submitted work. Piotr Ponikowski reports personal fees from Vifor Pharma and personal fees from AMGEN, outside the submitted work. Ewa A. Jankowska reports personal fees from Vifor Pharma and FRESENIUS, outside the submitted work.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

