Identification and validation of the role of matrix metalloproteinase-1 in cervical cancer
- PMID: 29436615
- PMCID: PMC5843389
- DOI: 10.3892/ijo.2018.4267
Identification and validation of the role of matrix metalloproteinase-1 in cervical cancer
Abstract
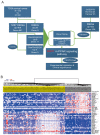
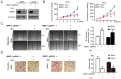
Lymph node (LN) metastasis at an early stage of cervical cancer is often an indicator of poor prognosis and is critical for subsequent adjuvant therapy. The current study aimed to identify aberrant gene signatures and biomarkers of metastasis for patients with cervical cancer. RNA-sequencing data of 132 LN negative (N0) and 60 LN positive (N1) cervical cancer samples obtained from The Cancer Genome Atlas database were analyzed. Differentially expressed genes were identified using R packages 'edgeR' and 'limma'. Kyoto Encyclopedia of Genes and Genomes pathway enrichment and Gene Set Enrichment Analysis (GSEA) were conducted. The GSE9750 dataset obtained from Gene Expression Omnibus was analyzed to identify genes that are persistently aberrantly expressed during the development of cervical cancer. The peroxisome proliferator-activated receptor (PPAR) signaling pathway was screened out to be significant during LN metastasis. In the two analyzed datasets, 11 genes were aberrantly expressed, while matrix metalloproteinase 1 (MMP1) was the only gene that was persistently overexpressed. Cell viability, wound healing and Transwell assays were performed to evaluate the effects of MMP1 knockdown in cervical cancer cell lines, and the expression of epithelial mesenchymal transition (EMT) markers was detected. Finally, the clinical significance of MMP1 was investigated. The current study identified that MMP1 was overexpressed and the PPAR signaling pathway was associated LN metastasis in patients with cervical cancer. Following knockdown of MMP1, the proliferation, migration and invasion of cervical cancer cell lines were weakened, the expression of epithelial marker E-cadherin was increased, and the expression of metastasis-associated gene vimentin was decreased. MMP1 was an independent prognostic factor for cervical cancer. The current study indicated that MMP1 has a key role in the regulation of cervical tumor growth and LN metastasis via EMT to a certain extent. The results suggest that MMP1 may be a biomarker for LN metastasis of cervical cancer, and further validation should be performed.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
ZEB1 promotes the progression and metastasis of cervical squamous cell carcinoma via the promotion of epithelial-mesenchymal transition.Int J Clin Exp Pathol. 2015 Sep 1;8(9):11258-67. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26617850 Free PMC article.
-
TRIM29 overexpression is associated with poor prognosis and promotes tumor progression by activating Wnt/β-catenin pathway in cervical cancer.Oncotarget. 2016 May 10;7(19):28579-91. doi: 10.18632/oncotarget.8686. Oncotarget. 2016. PMID: 27081037 Free PMC article.
-
Identification of crucial aberrantly methylated and differentially expressed genes related to cervical cancer using an integrated bioinformatics analysis.Biosci Rep. 2020 May 29;40(5):BSR20194365. doi: 10.1042/BSR20194365. Biosci Rep. 2020. PMID: 32368784 Free PMC article.
-
Semaphorin 4D expression is associated with a poor clinical outcome in cervical cancer patients.Microvasc Res. 2014 May;93:1-8. doi: 10.1016/j.mvr.2014.02.007. Epub 2014 Mar 3. Microvasc Res. 2014. PMID: 24603190
-
ΔNp63α attenuates tumor aggressiveness by suppressing miR-205/ZEB1-mediated epithelial-mesenchymal transition in cervical squamous cell carcinoma.Tumour Biol. 2016 Aug;37(8):10621-32. doi: 10.1007/s13277-016-4921-5. Epub 2016 Feb 10. Tumour Biol. 2016. PMID: 26864590
Cited by
-
Expression of collagenases (matrix metalloproteinase-1, -8, -13) and tissue inhibitor of metalloproteinase-3 (TIMP-3) in naturally occurring bovine cutaneous fibropapillomas.Front Vet Sci. 2023 Jan 12;9:1072672. doi: 10.3389/fvets.2022.1072672. eCollection 2022. Front Vet Sci. 2023. PMID: 36713871 Free PMC article.
-
The HPV-18 E7 CKII phospho acceptor site is required for maintaining the transformed phenotype of cervical tumour-derived cells.PLoS Pathog. 2019 May 22;15(5):e1007769. doi: 10.1371/journal.ppat.1007769. eCollection 2019 May. PLoS Pathog. 2019. PMID: 31116803 Free PMC article.
-
Gene Expression Analysis Identifies Novel Targets for Cervical Cancer Therapy.Front Immunol. 2018 Sep 19;9:2102. doi: 10.3389/fimmu.2018.02102. eCollection 2018. Front Immunol. 2018. PMID: 30283446 Free PMC article.
-
Proteases and HPV-Induced Carcinogenesis.Cancers (Basel). 2022 Jun 21;14(13):3038. doi: 10.3390/cancers14133038. Cancers (Basel). 2022. PMID: 35804810 Free PMC article. Review.
-
The Role of Peroxisome Proliferator-Activated Receptors in Endometrial Cancer.Int J Mol Sci. 2023 May 24;24(11):9190. doi: 10.3390/ijms24119190. Int J Mol Sci. 2023. PMID: 37298140 Free PMC article. Review.
References
-
- Munro A, Codde J, Spilsbury K, Steel N, Stewart CJ, Salfinger SG, Tan J, Mohan GR, Leung Y, Semmens JB, et al. Risk of persistent and recurrent cervical neoplasia following incidentally detected adenocarcinoma in situ. Am J Obstet Gynecol. 2017;216:272.e1–272.e7. doi: 10.1016/j.ajog.2016.11.1044. - DOI - PubMed
-
- Kim S-W, Chun M, Ryu H-S, Chang SJ, Kong TW, Lee EJ, Lee YH, Oh YT. Salvage radiotherapy with or without concurrent chemotherapy for pelvic recurrence after hysterectomy alone for early-stage uterine cervical cancer. Strahlenther Onkol. 2017;193:534–542. doi: 10.1007/s00066-017-1122-0. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

