Adverse events linked with the use of chimeric and humanized anti-CD20 antibodies in children with idiopathic nephrotic syndrome
- PMID: 29436729
- PMCID: PMC5980330
- DOI: 10.1111/bcp.13548
Adverse events linked with the use of chimeric and humanized anti-CD20 antibodies in children with idiopathic nephrotic syndrome
Abstract
Aims: Anti-CD20 antibodies are increasingly being used to treat idiopathic nephrotic syndrome (INS) in children. While they may allow steroid and calcineurin inhibitor withdrawal, repeated infusions of anti-CD20 antibodies are often required to maintain remission. Data on their potential toxicity in INS are needed, to consider repeated infusions.
Methods: We investigated the side effects associated with the use of rituximab (a chimeric antibody; 130 patients) and ofatumumab (a humanized antibody; 37 patients) in children with INS (steroid-dependent and steroid/calcineurin inhibitor-dependent disease) treated at a national referral centre over a 9-year period (400 treatments; follow-up 1-9 years).
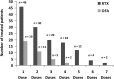
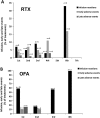
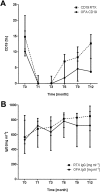
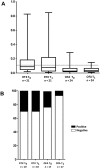
Results: Infusion reactions were mainly absent in children with steroid-dependent disease. Rash, dyspnoea, fever, cough and itchy throat (5% and 18% following rituximab and ofatumumab infusion, respectively) were resolved by using premedication with salbutamol. Other short-term reactions (up to 3 months), including arthritis (2%) and lung injury (1%), were more common with rituximab. Infections were observed 3-9 months following infusion, were similarly common in the two groups and resolved with targeted therapies [antibiotic, fluconazole, immunoglobulins (Igs), etc.]. The number of circulating CD19/20 cells fell to 0 at month 1 and were reconstituted at month 3; circulating IgG antibodies remained within the normal range for 1 year. Tetanus and hepatitis B virus immunization was not modified by either treatment; Epstein-Barr virus and John Cunningham virus activation markers were occasionally observed.
Conclusion: Overall, the toxicity of anti-CD20 monoclonal antibodies was limited to post-infusion side effects in children with more complex disease. The relatively safe profile of anti-CD20 antibodies supports their use as steroid-sparing agents in children with INS.
Keywords: nephrotic syndrome; ofatumumab; rituximab; side effects.
© 2018 The British Pharmacological Society.
Figures
References
-
- Du J, Yang H, Guo Y, Ding J. Structure of the Fab fragment of therapeutic antibody ofatumumab provides insights into the recognition mechanism with CD20. Mol Immunol 2009; 46: 2419–2423. - PubMed
-
- Furtado M, Dyer MJ, Johnson R, Berrow M, Rule S. Ofatumumab monotherapy in relapsed/refractory mantle cell lymphoma‐‐a phase II trial. Br J Haematol 2014; 165: 575–578. - PubMed
-
- Taylor PC, Quattrocchi E, Mallett S, Kurrasch R, Petersen J, Chang DJ. Ofatumumab, a fully human anti‐CD20 monoclonal antibody, in biological‐naive, rheumatoid arthritis patients with an inadequate response to methotrexate: a randomised, double‐blind, placebo‐controlled clinical trial. Ann Rheum Dis 2011; 70: 2119–2125. - PMC - PubMed
-
- Sorensen PS, Lisby S, Grove R, Derosier F, Shackelford S, Havrdova E, et al Safety and efficacy of ofatumumab in relapsing‐remitting multiple sclerosis: a phase 2 study. Neurology 2014; 82: 573–581. - PubMed
-
- Basu B. Ofatumumab for rituximab‐resistant nephrotic syndrome. N Engl J Med 2014; 370: 1268–1270. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources

