Alirocumab vs usual lipid-lowering care as add-on to statin therapy in individuals with type 2 diabetes and mixed dyslipidaemia: The ODYSSEY DM-DYSLIPIDEMIA randomized trial
- PMID: 29436756
- PMCID: PMC5969299
- DOI: 10.1111/dom.13257
Alirocumab vs usual lipid-lowering care as add-on to statin therapy in individuals with type 2 diabetes and mixed dyslipidaemia: The ODYSSEY DM-DYSLIPIDEMIA randomized trial
Abstract
Aim: To compare alirocumab, a proprotein convertase subtilisin-kexin type 9 inhibitor, with usual care (UC) in individuals with type 2 diabetes (T2DM) and mixed dyslipidaemia not optimally managed by maximally tolerated statins in the ODYSSEY DM-DYSLIPIDEMIA trial (NCT02642159).
Materials and methods: The UC options (no additional lipid-lowering therapy; fenofibrate; ezetimibe; omega-3 fatty acid; nicotinic acid) were selected prior to stratified randomization to open-label alirocumab 75 mg every 2 weeks (with increase to 150 mg every 2 weeks at week 12 if week 8 non-HDL cholesterol concentration was ≥2.59 mmol/L [100 mg/dL]) or UC for 24 weeks. The primary efficacy endpoint was percentage change in non-HDL cholesterol from baseline to week 24.
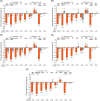
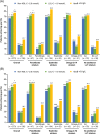
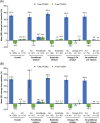
Results: The randomized population comprised 413 individuals (intention-to-treat population, n = 409; safety population, n = 412). At week 24, the mean non-HDL cholesterol reductions were superior with alirocumab (-32.5% difference vs UC, 97.5% confidence interval -38.1 to -27.0; P < .0001). Overall, 63.6% of alirocumab-treated individuals were maintained on 75 mg every 2 weeks. Alirocumab also reduced LDL cholesterol (-43.0%), apolipoprotein B (-32.3%), total cholesterol (-24.6%) and LDL particle number (-37.8%) at week 24 vs UC (all P < .0001). Consistent with the overall trial comparison, alirocumab reduced non-HDL cholesterol to a greater degree within each UC stratum at week 24. The incidence of treatment-emergent adverse events was 68.4% (alirocumab) and 66.4% (UC). No clinically meaningful effect on glycated haemoglobin, or change in number of glucose-lowering agents, was seen.
Conclusions: In individuals with T2DM and mixed dyslipidaemia on maximally tolerated statin, alirocumab showed superiority to UC in non-HDL cholesterol reduction and was generally well tolerated.
Keywords: PCSK9; mixed dyslipidaemia; non-HDL cholesterol; type 2 diabetes.
© 2018 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
K.K.R. has received: personal fees (data safety monitoring board) from AbbVie, Inc.; consultant fees/honoraria from Aegerion, Algorithm, Amgen, AstraZeneca, Boehringer Ingelheim, Cerenis, Eli Lilly and Company, Ionis Pharmaceuticals, Kowa, Medicines Company, MSD, Novartis, Pfizer, Inc., Regeneron Pharmaceuticals, Inc., Resverlogix, Sanofi and Takeda; and research grants from Kowa, Pfizer, Inc., and Regeneron Pharmaceuticals, Inc. L.A.L. has received: personal fees from Esperion; grants and personal fees from Amgen, AstraZeneca, Eli Lilly and Company, Merck, Regeneron Pharmaceuticals, Inc. and Sanofi; and grants from Kowa and the Medicine Company. D.M.‐W. has received speaker's bureau and consultant/advisory board fees from Amgen, AstraZeneca, Boehringer Ingelheim, MSD (Merck), Novartis, Novo Nordisk and Sanofi. B.C. has received: research funding and personal fees from Sanofi and Regeneron Pharmaceuticals, Inc. during the conduct of the study; research funding from Pfizer, Inc.; and honoraria from AstraZeneca, Pierre Fabre, Janssen, Eli Lilly and Company, MSD Merck & Co., Novo Nordisk, Sanofi and Takeda. H.M.C. has received grants, personal fees and non‐financial support from Sanofi and Regeneron Pharmaceuticals, Inc. during the conduct of the study; grants, personal fees and non‐financial support from Eli Lilly and Company; grants and other support from Roche Pharmaceuticals; grants from Pfizer, Inc., Boehringer Ingelheim, and AstraZeneca LP; and other support from Bayer. R.R.H. has received research funding from AstaMed, Eli Lilly and Company, Hitachi, Lexicon, Novo Nordisk and Viacyte and is a consultant for and/or advisory panel member of Alere, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Elcelyx, Gilead, Intarcia, Ionis, Janssen/Johnson & Johnson, Merck and Sanofi‐Aventis. F.J.T. has received speaker's bureau and consultant/advisory board fees from AstraZeneca, Amgen, Boehringer Ingelheim, Bristol‐Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, Janssen Pharmaceuticals, Merck Sharpe & Dohme, Novartis Pharmaceuticals Co., Novo Nordisk, Sanofi and Regeneron Pharmaceuticals, Inc. C.D., M.B.‐B. and A.L. are employees of and shareholders in Sanofi. R.S. is an employee of and shareholder in Regeneron Pharmaceuticals, Inc. S.D.P. has received research funding from AstraZeneca, Boheringer Ingelheim, Novartis Pharmaceuticals Co. and Merck Sharpe & Dohme; and is a consultant for or has received honoraria from AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, GlaxoSmithKline, Janssen Pharmaceuticals, Laboratoires Servier, Merck Sharpe & Dohme, Novartis Pharmaceuticals Co., Novo Nordisk, Sanofi, Servier and Takeda Pharmaceuticals.
Figures

References
-
- American Diabetes Association . 9. Cardiovascular disease and risk management. Diabetes Care. 2017;40:S75‐S87. - PubMed
-
- Ryden L, Grant PJ, Anker SD, et al. ESC guidelines on diabetes, pre‐diabetes, and cardiovascular diseases developed in collaboration with the EASD: the task force on diabetes, pre‐diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J. 2013;34:3035‐3087. - PubMed
-
- Bays HE, Jones PH, Orringer CE, Brown WV, Jacobson TA. National lipid association annual summary of clinical lipidology 2016. J Clin Lipidol. 2016;10:S1‐43. - PubMed
-
- Catapano AL, Graham I, De Backer G, et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016;37:2999‐3058. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

