An anti-TL1A antibody for the treatment of asthma and inflammatory bowel disease
- PMID: 29436901
- PMCID: PMC5973687
- DOI: 10.1080/19420862.2018.1440164
An anti-TL1A antibody for the treatment of asthma and inflammatory bowel disease
Abstract
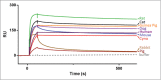
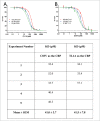
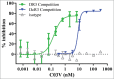
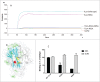
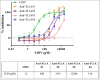
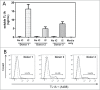
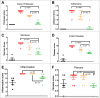
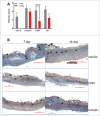
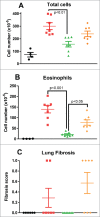
TL1A is an attractive therapeutic target for the treatment of mucosal inflammation associated with inflammatory bowel disease (IBD) and asthma. Blockade of the TL1A pathway has been shown to reduce inflammatory responses while leaving baseline immunity intact, and to be beneficial in animal models of colitis and asthma. Given the therapeutic potential of blocking this pathway in IBD and asthma, we developed C03V, a human antibody that binds with high affinity to soluble and membrane-bound TL1A. In an assay measuring apoptosis induced by exogenous TL1A, C03V was 43-fold more potent than the next most potent anti-TL1A antibody analyzed. C03V also potently inhibited endogenous TL1A activity in a primary cell-based assay. This potency was linked to the C03V-binding epitope on TL1A, encompassing the residue R32. This residue is critical for the binding of TL1A to its signaling receptor DR3 but not to its decoy receptor DcR3, and explains why C03V inhibited TL1A-DR3 binding to a much greater extent than TL1A-DcR3 binding. This characteristic may be advantageous to preserve some of the homeostatic functions of DcR3, such as TL1A antagonism. In colitis models, C03V significantly ameliorated microscopic, macroscopic and clinical aspects of disease pathology, and in an asthma model it significantly reduced airways inflammation. Notable in both types of disease model was the reduction in fibrosis observed after C03V treatment. C03V has the potential to address unmet medical needs in asthma and IBD.
Keywords: C03V; DR3; DcR3; TL1A; antibody; asthma; fully human; inflammatory bowel disease.
Figures


References
-
- Bamias G, 3rd Martin C, Marini M, Hoang S, Mishina M, Ross WG, Sachedina MA, Friel CM, Mize J, Bickston SJ, et al.. Expression, localization, and functional activity of TL1A, a novel Th1-polarizing cytokine in inflammatory bowel disease. . 2003;171:4868–74. doi:10.4049/jimmunol.171.9.4868. PMID:14568967. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
