Targeting mitochondrial responses to intra-articular fracture to prevent posttraumatic osteoarthritis
- PMID: 29437147
- PMCID: PMC5987523
- DOI: 10.1126/scitranslmed.aan5372
Targeting mitochondrial responses to intra-articular fracture to prevent posttraumatic osteoarthritis
Abstract
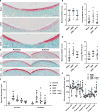
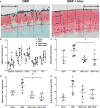
We tested whether inhibiting mechanically responsive articular chondrocyte mitochondria after severe traumatic injury and preventing oxidative damage represent a viable paradigm for posttraumatic osteoarthritis (PTOA) prevention. We used a porcine hock intra-articular fracture (IAF) model well suited to human-like surgical techniques and with excellent anatomic similarities to human ankles. After IAF, amobarbital or N-acetylcysteine (NAC) was injected to inhibit chondrocyte electron transport or downstream oxidative stress, respectively. Effects were confirmed via spectrophotometric enzyme assays or glutathione/glutathione disulfide assays and immunohistochemical measures of oxidative stress. Amobarbital or NAC delivered after IAF provided substantial protection against PTOA at 6 months, including maintenance of proteoglycan content, decreased histological disease scores, and normalized chondrocyte metabolic function. These data support the therapeutic potential of targeting chondrocyte metabolism after injury and suggest a strong role for mitochondria in mediating PTOA.
Copyright © 2018 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures





References
-
- Brown TD, Johnston RC, Saltzman CL, Marsh JL, Buckwalter JA. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. J Orthop Trauma. 2006 Nov-Dec;20(10):739–44. - PubMed
-
- Praemer A, Furner S, Furner RD. The Burden of Musculoskeletal Diseases in the United States: Prevalence, Societal and Economic Cost. AAOS; 1999.
-
- Marsh JL, Buckwalter JA, Gelberman R, Dirschl D, Olson S, Brown T, Llinias A. Articular fractures: does an anatomic reduction really change the result? J Bone Joint Surg Am. 2002 Jul;84-a(7):1259–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

