Single-agent ibrutinib in treatment-naïve and relapsed/refractory chronic lymphocytic leukemia: a 5-year experience
- PMID: 29437592
- PMCID: PMC5921964
- DOI: 10.1182/blood-2017-10-810044
Single-agent ibrutinib in treatment-naïve and relapsed/refractory chronic lymphocytic leukemia: a 5-year experience
Abstract
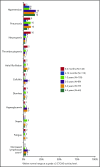
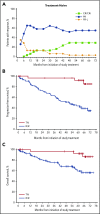
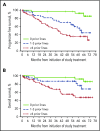
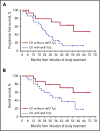
We previously reported durable responses and manageable safety of ibrutinib from a 3-year follow-up of treatment-naïve (TN) older patients (≥65 years of age) and relapsed/refractory (R/R) patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL). We now report on long-term efficacy and safety with median follow-up of 5 years in this patient population with TN (N = 31) and R/R (N = 101) CLL/SLL. With the current 5-year follow-up, ibrutinib continues to yield a high overall response rate of 89%, with complete response rates increasing over time to 29% in TN patients and 10% in R/R patients. The median progression-free survival (PFS) was not reached in TN patients. The 5-year PFS rate was 92% in TN patients and 44% in R/R patients. Median PFS in R/R patients was 51 months; in those with del(11q), del(17p), and unmutated IGHV, it was 51, 26, and 43 months, respectively, demonstrating long-term efficacy of ibrutinib in some high-risk subgroups. Survival outcomes were less robust for R/R patients with del(17p) and those who received more prior therapies. The onset of grade ≥3 cytopenias, such as neutropenia and thrombocytopenia, decreased over time. Treatment--limiting adverse events were more frequent during the first year compared with subsequent periods. These results demonstrate sustained efficacy and acceptable tolerability of ibrutinib over an extended time, providing the longest experience for Bruton tyrosine kinase inhibitor treatment in patients with CLL/SLL. These trials were registered at www.clinicaltrials.gov as #NCT01105247 and #NCT01109069.
© 2018 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: S.O. holds a consultancy/advisory role and received honoraria from AbbVie, Janssen, and Pharmacyclics LLC, an AbbVie Company, and received research funding from Pharmacyclics LLC, an AbbVie Company. R.R.F. received honoraria, holds a consultancy/advisory role, and received travel accommodations and expenses from Pharmacyclics LLC, an AbbVie Company and AbbVie, and served on a speakers bureau for Pharmacyclics LLC, an AbbVie Company. S.C. holds a consultancy/advisory role for Pharmacyclics LLC, an AbbVie Company, AbbVie, Gilead, Novartis, Janssen, and Celgene, and received research funding from Gilead, Celgene, Novartis, AbbVie, and Pharmacyclics LLC, an AbbVie Company. I.W.F. received research funding from Genentech, Janssen, and Pharmacyclics LLC, an AbbVie Company. J.A.B. received honoraria, holds a consultancy/advisory role and received travel accommodations and expenses from Gilead, TG Therapeutics, Pharmacyclics LLC, an AbbVie Company, Novartis, and Janssen, and received research funding from Pharmacyclics LLC, an AbbVie Company. K.B. received research funding from Celgene, Novartis, Janssen, Pharmacyclics LLC, an AbbVie Company, Seattle Genetics, Millennium, Gilead, Morphosys, and Constellation Pharmaceutical. J.S. holds a consultancy/advisory role and received research funding from Gilead, Pharmacyclics LLC, an AbbVie Company, Celgene, AbbVie, Genentech, Acerta, and TG Therapeutics. W.W. received honoraria from Sanofi, Genentech/Roche, Pharmacyclics LLC, an AbbVie Company, Celgene, Gilead, GSK/Novartis, Genzyme, Merck, AbbVie, and Emergent; received research funding from GSK/Novartis, AbbVie, Genentech, Karyopharm, Pharmacyclics LLC, an AbbVie Company, Acerta, Gilead, Janssen, Emergent, Juno, and Kite; and holds a consulting/advisory role with Sanofi, Genentech/Roche, Pharmacyclics LLC, an AbbVie Company, Celgene, Gilead, GSK/Novartis, Genzyme, Merck, AbbVie, and Emergent. J.J. is employed with Celgene; received honoraria from Janssen and Acerta; holds a consultancy/advisory role with Pharmacyclics LLC, an AbbVie Company, Janssen, AbbVie, Morphosys, and Gilead; and received research funding from Pharmacyclics LLC, an AbbVie Company, AbbVie, Janssen, Genentech, Gilead, and Acerta. Y.L. is employed with Pharmacyclics LLC, an AbbVie Company; holds stock ownership with AbbVie; and has received travel accommodations and expenses from Pharmacyclics LLC, an AbbVie Company. D.F.J. is employed with Pharmacyclics LLC, an AbbVie Company; holds stock ownership with AbbVie; and has patents with AbbVie. A.D.C. is employed with Pharmacyclics LLC, an AbbVie Company, and holds stock ownership with AbbVie. J.C.B. received research funding from Genentech, Acerta, and Pharmacyclics LLC, an AbbVie Company. The remaining authors declare no competing financial interests.
Figures



Comment in
-
Ibrutinib: coming of age?Blood. 2018 Apr 26;131(17):1880-1882. doi: 10.1182/blood-2018-02-832071. Blood. 2018. PMID: 29699993 No abstract available.
-
Ibrutinib and fungus: an invasive concern.Blood. 2018 Apr 26;131(17):1882-1884. doi: 10.1182/blood-2018-02-832154. Blood. 2018. PMID: 29699994 No abstract available.
References
-
- Catovsky D, Richards S, Matutes E, et al. ; NCRI Chronic Lymphocytic Leukaemia Working Group. Assessment of fludarabine plus cyclophosphamide for patients with chronic lymphocytic leukaemia (the LRF CLL4 Trial): a randomised controlled trial. Lancet. 2007;370(9583):230-239. - PubMed
-
- Flinn IW, Neuberg DS, Grever MR, et al. Phase III trial of fludarabine plus cyclophosphamide compared with fludarabine for patients with previously untreated chronic lymphocytic leukemia: US Intergroup Trial E2997. J Clin Oncol. 2007;25(7):793-798. - PubMed
-
- Hallek M, Fischer K, Fingerle-Rowson G, et al. ; German Chronic Lymphocytic Leukaemia Study Group. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376(9747):1164-1174. - PubMed
-
- Fischer K, Bahlo J, Fink AM, et al. Long-term remissions after FCR chemoimmunotherapy in previously untreated patients with CLL: updated results of the CLL8 trial. Blood. 2016;127(2):208-215. - PubMed
-
- Keating MJ, O’Brien S, Albitar M, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23(18):4079-4088. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

