α1-Antitrypsin infusion for treatment of steroid-resistant acute graft-versus-host disease
- PMID: 29437593
- PMCID: PMC5865235
- DOI: 10.1182/blood-2017-11-815746
α1-Antitrypsin infusion for treatment of steroid-resistant acute graft-versus-host disease
Abstract
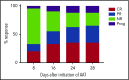
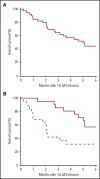
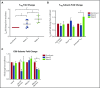
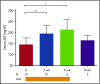
Corticosteroid resistance after acute graft-versus-host disease (SR-aGVHD) results in high morbidity and mortality after allogeneic hematopoietic cell transplantation. Current immunosuppressive therapies for SR-aGVHD provide marginal effectiveness because of poor response or excessive toxicity, primarily from infection. α1-Antitrypsin (AAT), a naturally abundant serine protease inhibitor, is capable of suppressing experimental GVHD by downmodulating inflammation and increasing ratios of regulatory (Treg) to effector T cells (Teffs). In this prospective multicenter clinical study, we sought to determine the safety and response rate of AAT administration in SR-aGVHD. Forty patients with a median age of 59 years received intravenous AAT twice weekly for 4 weeks as first-line treatment of SR-aGVHD. The primary end point was overall response rate (ORR), the proportion of patients with SR-aGVHD in complete (CR) or partial response by day 28 without addition of further immunosuppression. Treatment was well tolerated without drug-related adverse events. A significant increase in serum levels of AAT was observed after treatment. The ORR and CR rates by day 28 were 65% and 35%, respectively, and included responses in all aGVHD target organs. At day 60, responses were sustained in 73% of patients without intervening immunosuppression. Infectious mortality was 10% at 6 months and 2.5% within 30 days of last AAT infusion. Consistent with preclinical data, correlative samples showed an increase in ratio of activated Tregs to Teffs after AAT treatment. These data suggest that AAT is safe and may be potentially efficacious in treating SR-aGVHD. This trial was registered at www.clinicaltrials.gov as #NCT01700036.
© 2018 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures
References
-
- MacMillan ML, DeFor TE, Weisdorf DJ. The best endpoint for acute GVHD treatment trials. Blood. 2010;115(26):5412-5417. - PubMed
-
- MacMillan ML, Weisdorf DJ, Wagner JE, et al. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: comparison of grading systems. Biol Blood Marrow Transplant. 2002;8(7):387-394. - PubMed
-
- MacMillan ML, Weisdorf DJ, Davies SM, et al. Early antithymocyte globulin therapy improves survival in patients with steroid-resistant acute graft-versus-host disease. Biol Blood Marrow Transplant. 2002;8(1):40-46. - PubMed
-
- Khoury H, Kashyap A, Adkins DR, et al. Treatment of steroid-resistant acute graft-versus-host disease with anti-thymocyte globulin. Bone Marrow Transplant. 2001;27(10):1059-1064. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

