Cardiovascular outcomes associated with canagliflozin versus other non-gliflozin antidiabetic drugs: population based cohort study
- PMID: 29437648
- PMCID: PMC5799855
- DOI: 10.1136/bmj.k119
Cardiovascular outcomes associated with canagliflozin versus other non-gliflozin antidiabetic drugs: population based cohort study
Abstract
Objective: To evaluate the cardiovascular safety of canagliflozin, a sodium-glucose cotransporter 2 inhibitor for the treatment of type 2 diabetes mellitus, in direct comparisons with DPP-4 inhibitors (DPP-4i), GLP-1 receptor agonists (GLP-1RA), or sulfonylureas, as used in routine practice.
Design: Population based retrospective cohort study.
Setting: Nationwide sample of patients with type 2 diabetes from a large de-identified US commercial healthcare database (Optum Clinformatics Datamart).
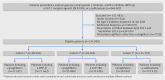
Participants: Three pairwise 1:1 propensity score matched cohorts of patients with type 2 diabetes 18 years and older who initiated canagliflozin or a comparator non-gliflozin antidiabetic agent (ie, a DPP-4i, a GLP-1RA, or a sulfonylurea) between April 2013 and September 2015.
Main outcome measures: The primary outcomes were heart failure admission to hospital and a composite cardiovascular endpoint (comprised of being admitted to hospital for acute myocardial infarction, ischemic stroke, or hemorrhagic stroke). Hazard ratios and 95% confidence intervals were estimated in each propensity score matched cohort controlling for more than 100 baseline characteristics.
Results: During a 30 month period, the hazard ratio for heart failure admission to hospital associated with canagliflozin was 0.70 (95% confidence interval 0.54 to 0.92) versus a DPP-4i (n=17 667 pairs), 0.61 (0.47 to 0.78) versus a GLP-1RA (20 539), and 0.51 (0.38 to 0.67) versus a sulfonylurea (17 354 ). The hazard ratio for the composite cardiovascular endpoint associated with canagliflozin was 0.89 (0.68 to 1.17) versus a DPP-4i, 1.03 (0.79 to 1.35) versus a GLP-1RA, and 0.86 (0.65 to 1.13) versus a sulfonylurea. Results were similar in sensitivity analyses further adjusting for baseline hemoglobin A1c levels and in subgroups of patients with and without prior cardiovascular disease or heart failure.
Conclusions: In this large cohort study, canagliflozin was associated with a lower risk of heart failure admission to hospital and with a similar risk of myocardial infarction or stroke in direct comparisons with three different classes of non-gliflozin diabetes treatment alternatives as used in routine care.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and have the following declarations. EP reports research grants from GSK and Boehringer-Ingelheim, not directly related to the topic of the submitted work. ABG reports grants from National Institutes of Health, American Diabetes Association, and Cleveland Clinic, other from National Institutes of Health, Diasome, Xoma, BAROnova, Caraco Pharmaceuticals, Amneal Pharmaceuticals, Lifescan, NovoNordisk, and Boston Heart Diagnostics, personal fees from Kowa, outside the submitted work. Work was performed during employment at Joslin Diabetes Center, now an employee of Novartis. SS is consultant to WHISCON LLC and to Aetion Inc, a software manufacturer of which he also owns equity. He is principal investigator of investigator-initiated grants to the Brigham and Women’s Hospital from Genentech, Bayer, Boehringer Ingelheim, US Food and Drug Administration, and Patient-Centered Outcomes Research Institute, not directly related to the topic of the submitted work. BME reports grants from Roche Diagnostics and Novartis Pharmaceuticals, consulting for Novartis Pharmaceuticals, Roche Diagnostics, Abbott Laboratories, US Food and Drug Administration, and UpToDate, outside the submitted work. BME serves as the co-chair of the American College of Cardiology's Task Force on Expert Clinical Decision Pathways Managing CV Disease Risk in Patients with Type 2 Diabetes. RJG reports grants from Pfizer, Novartis, and Kowa outside the submitted work. SCK reports grants from Pfizer Inc, AstraZeneca, Bristol-Myers Squibb, Merck, and Genentech, outside the submitted work.
Figures
References
-
- MacDonald MR, Petrie MC, Varyani F, et al. CHARM Investigators Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: an analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) programme. Eur Heart J 2008;29:1377-85. 10.1093/eurheartj/ehn153 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous