Altered Repolarization Reserve in Failing Rabbit Ventricular Myocytes: Calcium and β-Adrenergic Effects on Delayed- and Inward-Rectifier Potassium Currents
- PMID: 29437761
- PMCID: PMC5813707
- DOI: 10.1161/CIRCEP.117.005852
Altered Repolarization Reserve in Failing Rabbit Ventricular Myocytes: Calcium and β-Adrenergic Effects on Delayed- and Inward-Rectifier Potassium Currents
Abstract
Background: Electrophysiological remodeling and increased susceptibility for cardiac arrhythmias are hallmarks of heart failure (HF). Ventricular action potential duration (APD) is typically prolonged in HF, with reduced repolarization reserve. However, underlying K+ current changes are often measured in nonphysiological conditions (voltage clamp, low pacing rates, cytosolic Ca2+ buffers).
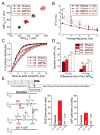
Methods and results: We measured the major K+ currents (IKr, IKs, and IK1) and their Ca2+- and β-adrenergic dependence in rabbit ventricular myocytes in chronic pressure/volume overload-induced HF (versus age-matched controls). APD was significantly prolonged only at lower pacing rates (0.2-1 Hz) in HF under physiological ionic conditions and temperature. However, when cytosolic Ca2+ was buffered, APD prolongation in HF was also significant at higher pacing rates. Beat-to-beat variability of APD was also significantly increased in HF. Both IKr and IKs were significantly upregulated in HF under action potential clamp, but only when cytosolic Ca2+ was not buffered. CaMKII (Ca2+/calmodulin-dependent protein kinase II) inhibition abolished IKs upregulation in HF, but it did not affect IKr. IKs response to β-adrenergic stimulation was also significantly diminished in HF. IK1 was also decreased in HF regardless of Ca2+ buffering, CaMKII inhibition, or β-adrenergic stimulation.
Conclusions: At baseline Ca2+-dependent upregulation of IKr and IKs in HF counterbalances the reduced IK1, maintaining repolarization reserve (especially at higher heart rates) in physiological conditions, unlike conditions of strong cytosolic Ca2+ buffering. However, under β-adrenergic stimulation, reduced IKs responsiveness severely limits integrated repolarizing K+ current and repolarization reserve in HF. This would increase arrhythmia propensity in HF, especially during adrenergic stress.
Keywords: action potential; calcium/calmodulin-dependent protein kinase II; electrophysiology; heart failure; potassium channels.
© 2018 American Heart Association, Inc.
Figures


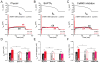
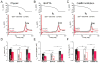

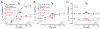
Comment in
-
Repolarization Reserve and Action Potential Dynamics in Failing Myocytes.Circ Arrhythm Electrophysiol. 2018 Feb;11(2):e006137. doi: 10.1161/CIRCEP.118.006137. Circ Arrhythm Electrophysiol. 2018. PMID: 29437765 Free PMC article. No abstract available.
References
-
- Tomaselli GF, Beuckelmann DJ, Calkins HG, Berger RD, Kessler PD, Lawrence JH, Kass D, Feldman AM, Marban E. Sudden cardiac death in heart failure. The role of abnormal repolarization. Circulation. 1994;90:2534–2539. - PubMed
-
- Watanabe E, Arakawa T, Uchiyama T, Tong M, Yasui K, Takeuchi H, Terasawa T, Kodama I, Hishida H. Prognostic significance of circadian variability of RR and QT intervals and QT dynamicity in patients with chronic heart failure. Heart Rhythm. 2007;4:999–1005. - PubMed
-
- Arsenos P, Gatzoulis KA, Dilaveris P, Gialernios T, Sideris S, Lazaros G, Archontakis S, Tsiachris D, Kartsagoulis E, Stefanadis C. The rate-corrected QT interval calculated from 24-hour holter recordings may serve as a significant arrhythmia risk stratifier in heart failure patients. Int J Cardiol. 2011;147:321–323. - PubMed
-
- Algra A, Tijssen JGP, Roelandt JRTC, Pool J, Lubsen J. QTc prolongation measured by standard 12-lead electrocardiography is an independent risk factor for sudden-death due to cardiac-arrest. Circulation. 1991;83:1888–1894. - PubMed
-
- Piccirillo G, Magri D, Matera S, Magnanti M, Torrini A, Pasquazzi E, Schifano E, Velitti S, Marigliano V, Quaglione R, Barilla F. QT variability strongly predicts sudden cardiac death in asymptomatic subjects with mild or moderate left ventricular systolic dysfunction: A prospective study. Eur Heart J. 2007;28:1344–1350. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

