T-Cell Exhaustion Signatures Vary with Tumor Type and Are Severe in Glioblastoma
- PMID: 29437767
- PMCID: PMC6081269
- DOI: 10.1158/1078-0432.CCR-17-1846
T-Cell Exhaustion Signatures Vary with Tumor Type and Are Severe in Glioblastoma
Abstract
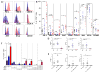
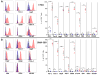
Purpose: T-cell dysfunction is a hallmark of glioblastoma (GBM). Although anergy and tolerance have been well characterized, T-cell exhaustion remains relatively unexplored. Exhaustion, characterized in part by the upregulation of multiple immune checkpoints, is a known contributor to failures amid immune checkpoint blockade, a strategy that has lacked success thus far in GBM. This study is among the first to examine, and credential as bona fide, exhaustion among T cells infiltrating human and murine GBM.Experimental Design: Tumor-infiltrating and peripheral blood lymphocytes (TILs and PBLs) were isolated from patients with GBM. Levels of exhaustion-associated inhibitory receptors and poststimulation levels of the cytokines IFNγ, TNFα, and IL2 were assessed by flow cytometry. T-cell receptor Vβ chain expansion was also assessed in TILs and PBLs. Similar analysis was extended to TILs isolated from intracranial and subcutaneous immunocompetent murine models of glioma, breast, lung, and melanoma cancers.Results: Our data reveal that GBM elicits a particularly severe T-cell exhaustion signature among infiltrating T cells characterized by: (1) prominent upregulation of multiple immune checkpoints; (2) stereotyped T-cell transcriptional programs matching classical virus-induced exhaustion; and (3) notable T-cell hyporesponsiveness in tumor-specific T cells. Exhaustion signatures differ predictably with tumor identity, but remain stable across manipulated tumor locations.Conclusions: Distinct cancers possess similarly distinct mechanisms for exhausting T cells. The poor TIL function and severe exhaustion observed in GBM highlight the need to better understand this tumor-imposed mode of T-cell dysfunction in order to formulate effective immunotherapeutic strategies targeting GBM. Clin Cancer Res; 24(17); 4175-86. ©2018 AACRSee related commentary by Jackson and Lim, p. 4059.
©2018 American Association for Cancer Research.
Conflict of interest statement
Figures




References
-
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–66. doi: 10.1016/S1470-2045(09)70025-7. - DOI - PubMed
-
- Kmiecik J, Poli A, Brons NH, Waha A, Eide GE, Enger PO, et al. Elevated CD3+ and CD8+ tumor-infiltrating immune cells correlate with prolonged survival in glioblastoma patients despite integrated immunosuppressive mechanisms in the tumor microenvironment and at the systemic level. Journal of neuroimmunology. 2013;264(1–2):71–83. doi: 10.1016/j.jneuroim.2013.08.013. - DOI - PubMed
-
- Dix AR, Brooks WH, Roszman TL, Morford LA. Immune defects observed in patients with primary malignant brain tumors. Journal of neuroimmunology. 1999;100(1–2):216–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

