Susceptibility to Hypertensive Renal Disease in the Spontaneously Hypertensive Rat Is Influenced by 2 Loci Affecting Blood Pressure and Immunoglobulin Repertoire
- PMID: 29437896
- PMCID: PMC5843527
- DOI: 10.1161/HYPERTENSIONAHA.117.10593
Susceptibility to Hypertensive Renal Disease in the Spontaneously Hypertensive Rat Is Influenced by 2 Loci Affecting Blood Pressure and Immunoglobulin Repertoire
Abstract
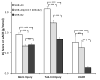
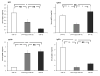
High blood pressure exerts its deleterious effects on health largely through acceleration of end-organ diseases. Among these, progressive loss of renal function is particularly important, not only for the direct consequences of kidney damage but also because loss of renal function is associated with amplification of other adverse cardiovascular outcomes. Genetic susceptibility to hypertension and associated end-organ disease is non-Mendelian in both humans and in a rodent model, the spontaneously hypertensive rat (SHR). Here, we report that hypertensive end-organ disease in the inbred SHR-A3 line is attributable to genetic variation in the immunoglobulin heavy chain on chromosome 6. This variation coexists with variation in a 10 Mb block on chromosome 17 that contains genetic variation in 2 genes involved in immunoglobulin Fc receptor signaling. Substitution of these genomic regions into the SHR-A3 genome from the closely related, but injury-resistant, SHR-B2 line normalizes both biomarker and histological measures of renal injury. Our findings indicate that genetic variation leads to a contribution by immune mechanisms hypertensive end-organ injury and that, in this rat model, disease is influenced by differences in germ line antibody repertoire.
Keywords: biomarkers; genetics; hypertension; immunoglobulin; proteinuria.
© 2018 American Heart Association, Inc.
Conflict of interest statement
None
Figures



Comment in
-
Genetic Susceptibility to Hypertension-Induced Renal Injury.Hypertension. 2018 Apr;71(4):559-560. doi: 10.1161/HYPERTENSIONAHA.118.10773. Epub 2018 Feb 5. Hypertension. 2018. PMID: 29437894 Free PMC article. No abstract available.
References
-
- Freedman BI, Soucie JM, McClellan WM. Family history of end-stage renal disease among incident dialysis patients. Journal of the American Society of Nephrology: JASN. 1997;8:1942–1945. - PubMed
-
- Freedman BI, Volkova NV, Satko SG, Krisher J, Jurkovitz C, Soucie JM, McClellan WM. Population-based screening for family history of end-stage renal disease among incident dialysis patients. Am J Nephrol. 2005;25:529–535. - PubMed
-
- Kottgen A, Glazer NL, Dehghan A, Hwang SJ, Katz R, Li M, Yang Q, Gudnason V, Launer LJ, Harris TB, Smith AV, Arking DE, Astor BC, Boerwinkle E, Ehret GB, Ruczinski I, Scharpf RB, Chen YD, de Boer IH, Haritunians T, Lumley T, Sarnak M, Siscovick D, Benjamin EJ, Levy D, Upadhyay A, Aulchenko YS, Hofman A, Rivadeneira F, Uitterlinden AG, van Duijn CM, Chasman DI, Pare G, Ridker PM, Kao WH, Witteman JC, Coresh J, Shlipak MG, Fox CS. Multiple loci associated with indices of renal function and chronic kidney disease. Nature Genetics. 2009;41:712–717. - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

