Magnetic resonance and photoacoustic imaging of brain tumor mediated by mesenchymal stem cell labeled with multifunctional nanoparticle introduced via carotid artery injection
- PMID: 29438105
- PMCID: PMC5863233
- DOI: 10.1088/1361-6528/aaaf16
Magnetic resonance and photoacoustic imaging of brain tumor mediated by mesenchymal stem cell labeled with multifunctional nanoparticle introduced via carotid artery injection
Abstract
Objective: To evaluate the feasibility of visualizing bone marrow-derived human mesenchymal stem cells (MSCs) labeled with a gold-coated magnetic resonance (MR)-active multifunctional nanoparticle and injected via the carotid artery for assessing the extent of MSC homing in glioma-bearing mice.
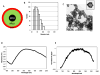
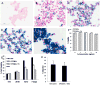
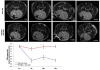
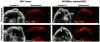
Materials and methods: Nanoparticles containing superparamagnetic iron oxide coated with gold (SPIO@Au) with a diameter of ∼82 nm and maximum absorbance in the near infrared region were synthesized. Bone marrow-derived MSCs conjugated with green fluorescent protein (GFP) were successfully labeled with SPIO@Au at 4 μg ml-1 and injected via the internal carotid artery in six mice bearing orthotopic U87 tumors. Unlabeled MSCs were used as a control. The ability of SPIO@Au-loaded MSCs to be imaged using MR and photoacoustic (PA) imaging at t = 0 h, 2 h, 24 h, and 72 h was assessed using a 7 T Bruker Biospec experimental MR scanner and a Vevo LAZR PA imaging system with a 5 ns laser as the excitation source. Histological analysis of the brain tissue was performed 72 h after MSC injection using GFP fluorescence, Prussian blue staining, and hematoxylin-and-eosin staining.
Results: MSCs labeled with SPIO@Au at 4 μg ml-1 did not exhibit cell death or any adverse effects on differentiation or migration. The PA signal in tumors injected with SPIO@Au-loaded MSCs was clearly more enhanced post-injection, as compared with the tumors injected with unlabeled MSCs at t = 72 h. Using the same mice, T2-weighted MR imaging results taken before injection and at t = 2 h, 24 h, and 72 h were consistent with the PA imaging results, showing significant hypointensity of the tumor in the presence of SPIO@Au-loaded MSCs. Histological analysis also showed co-localization of GFP fluorescence and iron, thereby confirming that SPIO@Au-labeled MSCs continue to carry their nanoparticle payloads even at 72 h after injection.
Conclusions: Our results demonstrated the feasibility of tracking carotid artery-injected SPIO@Au-labeled MSCs in vivo via MR and PA imaging.
Figures


References
-
- Miletic H, et al. Bystander killing of malignant glioma by bone marrow-derived tumor-infiltrating progenitor cells expressing a suicide gene. Mol Ther. 2007;15:1373–81. - PubMed
-
- Nakamizo A, et al. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65:3307–18. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
