Sjögren-Like Lacrimal Keratoconjunctivitis in Germ-Free Mice
- PMID: 29438346
- PMCID: PMC5855787
- DOI: 10.3390/ijms19020565
Sjögren-Like Lacrimal Keratoconjunctivitis in Germ-Free Mice
Abstract
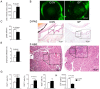
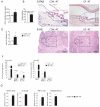
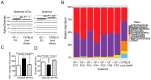
Commensal bacteria play an important role in the formation of the immune system but their role in the maintenance of immune homeostasis at the ocular surface and lacrimal gland remains poorly understood. This study investigated the eye and lacrimal gland phenotype in germ-free and conventional C57BL/6J mice. Our results showed that germ-free mice had significantly greater corneal barrier disruption, greater goblet cell loss, and greater total inflammatory cell and CD4⁺ T cell infiltration within the lacrimal gland compared to the conventionally housed group. A greater frequency of CD4⁺IFN-γ⁺ cells was observed in germ-free lacrimal glands. Females exhibited a more severe phenotype compared to males. Adoptive transfer of CD4⁺ T cells isolated from female germ-free mice into RAG1KO mice transferred Sjögren-like lacrimal keratoconjunctivitis. Fecal microbiota transplant from conventional mice reverted dry eye phenotype in germ-free mice and decreased CD4⁺IFN-γ⁺ cells to levels similar to conventional C57BL/6J mice. These findings indicate that germ-free mice have a spontaneous lacrimal keratoconjunctivitis similar to that observed in Sjögren syndrome patients and demonstrate that commensal bacteria function in maintaining immune homeostasis on the ocular surface. Thus, manipulation of intestinal commensal bacteria has the potential to become a novel therapeutic approach to treat Sjögren Syndrome.
Keywords: Sjögren syndrome; commensal bacteria; dry eye; fecal transplant; germ-free mice; goblet cell.
Conflict of interest statement
All authors state that they have no conflicts of interest.
Figures



References
-
- Schabereiter-Gurtner C., Maca S., Rolleke S., Nigl K., Lukas J., Hirschl A., Lubitz W., Barisani-Asenbauer T. 16S rDNA-based identification of bacteria from conjunctival swabs by PCR and DGGE fingerprinting. Investig. Ophthalmol. Vis. Sci. 2001;42:1164–1171. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

