Efficacy of an Optimised Bacteriophage Cocktail to Clear Clostridium difficile in a Batch Fermentation Model
- PMID: 29438355
- PMCID: PMC5872124
- DOI: 10.3390/antibiotics7010013
Efficacy of an Optimised Bacteriophage Cocktail to Clear Clostridium difficile in a Batch Fermentation Model
Abstract
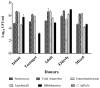
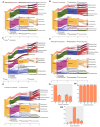
Clostridium difficile infection (CDI) is a major cause of infectious diarrhea. Conventional antibiotics are not universally effective for all ribotypes, and can trigger dysbiosis, resistance and recurrent infection. Thus, novel therapeutics are needed to replace and/or supplement the current antibiotics. Here, we describe the activity of an optimised 4-phage cocktail to clear cultures of a clinical ribotype 014/020 strain in fermentation vessels spiked with combined fecal slurries from four healthy volunteers. After 5 h, we observed ~6-log reductions in C. difficile abundance in the prophylaxis regimen and complete C. difficile eradication after 24 h following prophylactic or remedial regimens. Viability assays revealed that commensal enterococci, bifidobacteria, lactobacilli, total anaerobes, and enterobacteria were not affected by either regimens, but a ~2-log increase in the enterobacteria, lactobacilli, and total anaerobe abundance was seen in the phage-only-treated vessel compared to other treatments. The impact of the phage treatments on components of the microbiota was further assayed using metagenomic analysis. Together, our data supports the therapeutic application of our optimised phage cocktail to treat CDI. Also, the increase in specific commensals observed in the phage-treated control could prevent further colonisation of C. difficile, and thus provide protection from infection being able to establish.
Keywords: Clostridium difficile; Clostridium difficile infection; bacteriophages; in vitro fermentation model; microbiome; phage therapy.
Conflict of interest statement
Although this work was funded by AmpliPhi Biosciences and under the terms of a license agreement to the University of Leicester headed by MRJC, the license agreement has since been terminated, and thus there is no conflict of interest in terms of license revenue shares. Similarly, all the other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Stewardson A.J., Allignol A., Beyersmann J., Graves N., Schumacher M., Meyer R., Tacconelli E., De Angelis G., Farina C., Pezzoli F., et al. The health and economic burden of bloodstream infections caused by antimicrobial-susceptible and non-susceptible Enterobacteriaceae and Staphylococcus aureus in European hospitals, 2010 and 2011: A multicentre retrospective cohort study. Euro Surveill. 2016;21 doi: 10.2807/1560-7917.ES.2016.21.33.30319. - DOI - PMC - PubMed
-
- Naylor N.R., Pouwels K.B., Hope R., Green N., Henderson K.L., Knight G.M., Atun R., Robotham J.V., Deeny S. A national estimate of the health and cost burden of Escherichia coli bacteraemia in the hospital setting: The importance of antibiotic resistance. bioRxiv. 2017 doi: 10.1101/153775. - DOI - PMC - PubMed
-
- O’Neil J. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. The Review on Antimicrobial Resistance 2014. [(accessed on 31 December 2017)]; Available online: https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Ta....
LinkOut - more resources
Full Text Sources
Other Literature Sources

