A phase I trial of the γ-secretase inhibitor MK-0752 in combination with gemcitabine in patients with pancreatic ductal adenocarcinoma
- PMID: 29438372
- PMCID: PMC5877439
- DOI: 10.1038/bjc.2017.495
A phase I trial of the γ-secretase inhibitor MK-0752 in combination with gemcitabine in patients with pancreatic ductal adenocarcinoma
Abstract
Background: The Notch pathway is frequently activated in cancer. Pathway inhibition by γ-secretase inhibitors has been shown to be effective in pre-clinical models of pancreatic cancer, in combination with gemcitabine.
Methods: A multi-centre, non-randomised Bayesian adaptive design study of MK-0752, administered per os weekly, in combination with gemcitabine administered intravenously on days 1, 8 and 15 (28 day cycle) at 800 or 1000 mg m-2, was performed to determine the safety of combination treatment and the recommended phase 2 dose (RP2D). Secondary and tertiary objectives included tumour response, plasma and tumour MK-0752 concentration, and inhibition of the Notch pathway in hair follicles and tumour.
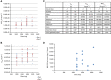
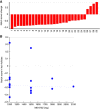
Results: Overall, 44 eligible patients (performance status 0 or 1 with adequate organ function) received gemcitabine and MK-0752 as first or second line treatment for pancreatic cancer. RP2Ds of MK-0752 and gemcitabine as single agents could be combined safely. The Bayesian algorithm allowed further dose escalation, but pharmacokinetic analysis showed no increase in MK-0752 AUC (area under the curve) beyond 1800 mg once weekly. Tumour response evaluation was available in 19 patients; 13 achieved stable disease and 1 patient achieved a confirmed partial response.
Conclusions: Gemcitabine and a γ-secretase inhibitor (MK-0752) can be combined at their full, single-agent RP2Ds.
Conflict of interest statement
DJ received support from Merck for meeting attendance and honoraria. Merck also contributed to some of the clinical trial costs associated with this study. DJ is a group leader in the Cancer Research UK funded CRUK Cambridge Institute, receiving core support from that Institute. MN is a full time employee of Merck, Kenilworth, NJ, USA. The remaining authors declare no conflict of interest.
Figures
References
-
- Bray SJ (2006) Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol 7: 678–689. - PubMed
-
- Burris HA 3RD, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15: 2403–2413. - PubMed
-
- Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, De La Fouchardiere C, Bennouna J, Bachet JB, Khemissa-Akouz F, Pere-Verge D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M Groupe Tumeurs Digestives of UnicancerPRODIGE Intergroup (2011) Folfirinox versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364: 1817–1825. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

