Oxidative stress increases M1dG, a major peroxidation-derived DNA adduct, in mitochondrial DNA
- PMID: 29438559
- PMCID: PMC5909422
- DOI: 10.1093/nar/gky089
Oxidative stress increases M1dG, a major peroxidation-derived DNA adduct, in mitochondrial DNA
Abstract
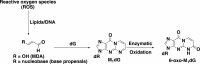
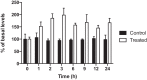
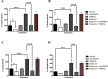
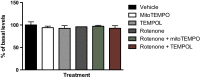
Reactive oxygen species (ROS) are formed in mitochondria during electron transport and energy generation. Elevated levels of ROS lead to increased amounts of mitochondrial DNA (mtDNA) damage. We report that levels of M1dG, a major endogenous peroxidation-derived DNA adduct, are 50-100-fold higher in mtDNA than in nuclear DNA in several different human cell lines. Treatment of cells with agents that either increase or decrease mitochondrial superoxide levels leads to increased or decreased levels of M1dG in mtDNA, respectively. Sequence analysis of adducted mtDNA suggests that M1dG residues are randomly distributed throughout the mitochondrial genome. Basal levels of M1dG in mtDNA from pulmonary microvascular endothelial cells (PMVECs) from transgenic bone morphogenetic protein receptor 2 mutant mice (BMPR2R899X) (four adducts per 106 dG) are twice as high as adduct levels in wild-type cells. A similar increase was observed in mtDNA from heterozygous null (BMPR2+/-) compared to wild-type PMVECs. Pulmonary arterial hypertension is observed in the presence of BMPR2 signaling disruptions, which are also associated with mitochondrial dysfunction and oxidant injury to endothelial tissue. Persistence of M1dG adducts in mtDNA could have implications for mutagenesis and mitochondrial gene expression, thereby contributing to the role of mitochondrial dysfunction in diseases.
Figures


References
-
- Elinav E., Nowarski R., Thaiss C.A., Hu B., Jin C., Flavell R.A.. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat. Rev. Cancer. 2013; 13:759–771. - PubMed
-
- St-Pierre J., Buckingham J.A., Roebuck S.J., Brand M.D.. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J. Biol. Chem. 2002; 277:44784–44790. - PubMed
-
- Marnett L.J. Oxyradicals and DNA damage. Carcinogenesis. 2000; 21:361–370. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

