Extracellular vesicles in the hematopoietic microenvironment
- PMID: 29439185
- PMCID: PMC5830368
- DOI: 10.3324/haematol.2017.183335
Extracellular vesicles in the hematopoietic microenvironment
Abstract
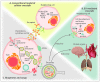
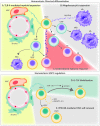
Self-renewal and differentiation are defining characteristics of hematopoietic stem and progenitor cells, and their balanced regulation is central to lifelong function of both blood and immune systems. In addition to cell-intrinsic programs, hematopoietic stem and progenitor cell fate decisions are subject to extrinsic cues from within the bone marrow microenvironment and systemically. Yet, many of the paracrine and endocrine mediators that shape hematopoietic function remain to be discovered. Extracellular vesicles serve as evolutionarily conserved, constitutive regulators of cell and tissue homeostasis, with several recent reports supporting a role for extracellular vesicles in the regulation of hematopoiesis. We review the physiological and pathophysiological effects that extracellular vesicles have on bone marrow compartmental function while highlighting progress in understanding vesicle biogenesis, cargo incorporation, differential uptake, and downstream effects of vesicle internalization. This review also touches on the role of extracellular vesicles in hematopoietic stem and progenitor cell fate regulation and recent advances in therapeutic and diagnostic applications of extracellular vesicles in hematologic disorders.
Copyright© 2018 Ferrata Storti Foundation.
Figures


References
-
- Blank U, Karlsson S. TGF-β signaling in the control of hematopoietic stem cells. Blood. 2015;125(23):3542–3550. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

