Synthesis and Fluorescent Property Study of Novel 1,8-Naphthalimide-Based Chemosensors
- PMID: 29439399
- PMCID: PMC6017435
- DOI: 10.3390/molecules23020376
Synthesis and Fluorescent Property Study of Novel 1,8-Naphthalimide-Based Chemosensors
Abstract
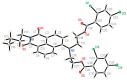
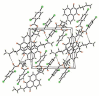
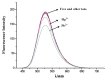
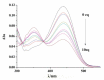
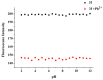
A series of novel mono- and di-substituted N-n-butyl-1,8-naphthalimide derivatives were synthesized simultaneously via a three-step reaction. The single crystal structure of N-n-butyl-4-[N',N'-bis(2',4'-dichlorobenzoyl)ethylamino]-1,8-naphthalimide (3f) was determined. The UV-vis and fluorescence properties of compound 3f were investigated. The 3f showed highly selective and sensitive fluorescence changes response towards Pb2+. A titration of monomer with Pb2+ ion was performed. When Pb2+ ion concentration increased from 0 to 10 eq., the fluorescent intensity of 3f decreased from 199.97 to 48.21. The pH effect on 3f showed that it is stable in a wide range of pH. The results indicated that 3f might be a probe molecule for Pb2+.
Keywords: N-n-Butyl-4-(N′,N′-dihydroxyethylamino)-1,8-naphthalimide; crystal structure; design; fluorescence; synthesis.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures






References
-
- Lu D.Q., Zhou L.Y., Wang R.W., Zhang X.B., He L., Zhang J., Hu X.X., Tan W.H. A two-photon fluorescent probe for endogenous superoxide anion radical detection and imaging in living cells and tissues. Sens. Actuators B Chem. 2017;250:259–266. doi: 10.1016/j.snb.2017.04.041. - DOI
-
- Sun J.Q., Ye B.F., Xia G.M., Zhao X.H., Wang H.M. A colorimetric and fluorescent chemosens or for the highly sensitive detection of CO2 gas: Experiment and DFT calculation. Sens. Actuators B Chem. 2016;233:76–82. doi: 10.1016/j.snb.2016.04.052. - DOI
-
- Hu X.X., Zheng X.L., Fan X.X., Su Y.T., Zhan X.Q., Zheng H. Semicarbazide-based naphthalimide as a highly selective and sensitive colorimetric and turn-on fluorescent chemodosimeter for Cu2+ Sens. Actuators B Chem. 2016;227:191–197. doi: 10.1016/j.snb.2015.12.037. - DOI
-
- Ge J., Geng X., Du Y.H., Chen J.J., Zhang L., Bai D.M., Ji D.Y., Hu Y.L., Li Z.H. Highly sensitive fluorescence detection of mercury (II) ions based on WS2, nanosheets and T7 exonuclease assisted cyclic enzymatic amplification. Sens. Actuators B Chem. 2017;249:189–194. doi: 10.1016/j.snb.2017.04.094. - DOI
-
- Sun J.Q., Ye B.F., Xia G.M., Wang H.M. A multi-responsive squaraine-based “turn on” fluorescent chemosensor for highly sensitive detection of Al3+, Zn2+, and Cd2+, in aqueous media and its biological application. Sens. Actuators B Chem. 2017;249:386–394. doi: 10.1016/j.snb.2017.03.134. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

