Chemical Sensing Applications of ZnO Nanomaterials
- PMID: 29439528
- PMCID: PMC5848984
- DOI: 10.3390/ma11020287
Chemical Sensing Applications of ZnO Nanomaterials
Abstract
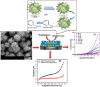
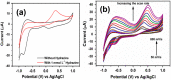
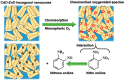
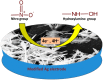
Recent advancement in nanoscience and nanotechnology has witnessed numerous triumphs of zinc oxide (ZnO) nanomaterials due to their various exotic and multifunctional properties and wide applications. As a remarkable and functional material, ZnO has attracted extensive scientific and technological attention, as it combines different properties such as high specific surface area, biocompatibility, electrochemical activities, chemical and photochemical stability, high-electron communicating features, non-toxicity, ease of syntheses, and so on. Because of its various interesting properties, ZnO nanomaterials have been used for various applications ranging from electronics to optoelectronics, sensing to biomedical and environmental applications. Further, due to the high electrochemical activities and electron communication features, ZnO nanomaterials are considered as excellent candidates for electrochemical sensors. The present review meticulously introduces the current advancements of ZnO nanomaterial-based chemical sensors. Various operational factors such as the effect of size, morphologies, compositions and their respective working mechanisms along with the selectivity, sensitivity, detection limit, stability, etc., are discussed in this article.
Keywords: chemical sensing; morphology; selectivity; sensitivity; synthesis; zinc oxide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



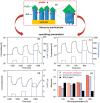
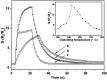
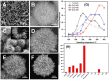

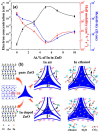



References
-
- Ebrahimiasl S., Seifi R., Nahli R.E., Azmi Zakaria A. Ppy/nanographene modified pencil graphite electrode nanosensor for detection and determination of herbicides in agricultural water. Sci. Adv. Mater. 2017;9:2045–2053.
-
- Xiang C., Wang Y., Liu H. A scientometrics review on nonpoint source pollution research. Ecol. Eng. 2017;99:400–408. doi: 10.1016/j.ecoleng.2016.11.028. - DOI
-
- Martinez D.E., Grondona S., Miglioranza K.S.B., Postigo C. Groundwater pollution sources, mechanisms, and prevention. Encycl. Anthr. 2018;5:87–96.
-
- Uma B.B., Uday S.P., Oinam G., Mondal A., Bandyopadhyay T.K., Tiwari O.N. Characterization, genetic regulation and production of cyanobacterial exopolysaccharides and its applicability for heavy metal removal. Carbohydr. Polym. 2018;179:228–243. - PubMed
-
- Song J.F., Lin Z.Z., Ge S., Li J., Qiu X.M., Zhou R.S., Li S.Z., Guo Z. Dispersible novel naphthalene-2,6-dicarboxylic acid monomethyl ester-based coordination polymers through in situ hydrolysis reaction: Highly sensitive detection of small molecules and metal ions. Sci. Adv. Mater. 2017;9:2054–2065.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

