HIPECT4: multicentre, randomized clinical trial to evaluate safety and efficacy of Hyperthermic intra-peritoneal chemotherapy (HIPEC) with Mitomycin C used during surgery for treatment of locally advanced colorectal carcinoma
- PMID: 29439668
- PMCID: PMC5812226
- DOI: 10.1186/s12885-018-4096-0
HIPECT4: multicentre, randomized clinical trial to evaluate safety and efficacy of Hyperthermic intra-peritoneal chemotherapy (HIPEC) with Mitomycin C used during surgery for treatment of locally advanced colorectal carcinoma
Abstract
Background: Local relapse and peritoneal carcinomatosis (PC) for pT4 colon cancer is estimated in 15,6% and 36,7% for 12 months and 36 months from surgical resection respectively, achieving a 5 years overall survival of 6%. There are promising results using prophylactic HIPEC in this group of patients, and it is estimated that up to 26% of all T4 colon cancer could benefit from this treatment with a minimal morbidity. Adjuvant HIPEC is effective to avoid the possibility of peritoneal seeding after surgical resection. Taking into account these results and the cumulative experience in HIPEC use, we will lead a randomized controlled trial to determine the effectiveness and safety of adjuvant treatment with HIPEC vs. standard treatment in patients with colon cancer at high risk of peritoneal recurrence (pT4).
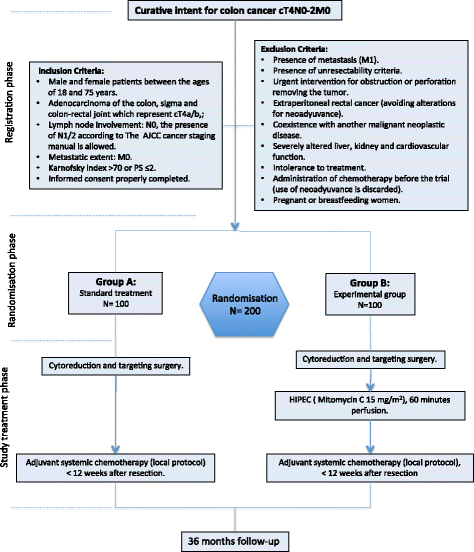
Methods/design: The aim of this study is to determine the effectiveness and safety of adjuvant HIPEC in preventing the development of PC in patients with colon cancer with a high risk of peritoneal recurrence (cT4). This study will be carried out in 15 Spanish HIPEC centres. Eligible for inclusion are patients who underwent curative resection for cT4NxM0 stage colon cancer. After resection of the primary tumour, 200 patients will be randomized to adjuvant HIPEC followed by routine adjuvant systemic chemotherapy in the experimental arm, or to systemic chemotherapy only in the control arm. Adjuvant HIPEC will be performed simultaneously after the primary resection. Mitomycin C will be used as chemotherapeutic agent, for 60 min at 42-43 °C. Primary endpoint is loco-regional control (LC) in months and the rate of loco-regional control (%LC) at 12 months and 36 months after resection.
Discussion: We assumed that adjuvant HIPEC will reduce the expected absolute risk of peritoneal recurrence from 36% to 18% at 36 months for T4 colon-rectal carcinoma.
Trial registration: NCT02614534 ( clinicaltrial.gov ) Nov-2015.
Keywords: Chemoprophylaxis; Colon carcinoma; HIPEC; Peritoneal carcinomatosis.
Conflict of interest statement
Ethics approval and consent to participate
Our study has been approved by Hospital University Reina Sofia, Cordoba ethics committee at 29th june 2015. Ref. 2841. Since this approval, all the other local ethics committes approved the protocol in each center included in this trial. The informed consent must be signed by participant previous complete information from the investigators.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
-
- Franko J, Shi Q, Goldman CD, Pockaj BA, Nelson GD, Goldberg RM, et al. Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: a pooled analysis of north central cancer treatment group phase III trials N9741 and N9841. J Clin Oncol. 2012;30:263–267. doi: 10.1200/JCO.2011.37.1039. - DOI - PMC - PubMed
-
- Snaebjornsson P, Coupe VMH, Jonasson L, Meijer GA, van Grieken NC, Jonasson JG. Stage II and III colon cancers carry the worst prognosis in a nationwide survival analysis. Shepherd's local peritoneal involvement revisited. Int. J. Cancer. 2014;135:467–78. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical