SeMet attenuates OTA-induced PCV2 replication promotion by inhibiting autophagy by activating the AKT/mTOR signaling pathway
- PMID: 29439710
- PMCID: PMC5812231
- DOI: 10.1186/s13567-018-0508-z
SeMet attenuates OTA-induced PCV2 replication promotion by inhibiting autophagy by activating the AKT/mTOR signaling pathway
Abstract
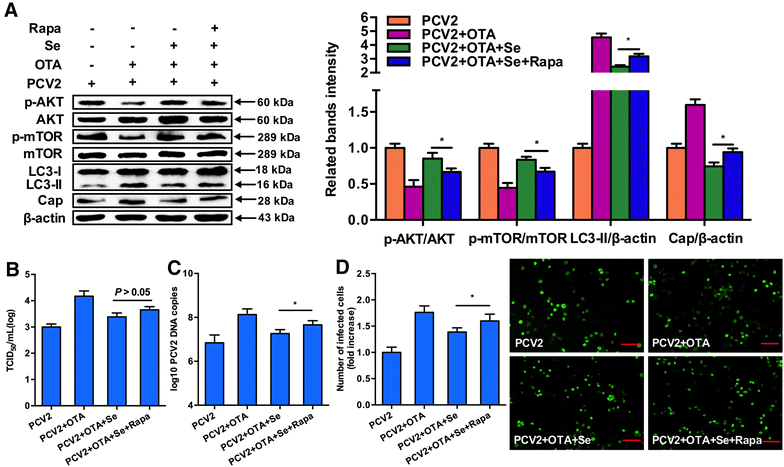
Porcine circovirus type 2 (PCV2) is recognized as the causative agent of porcine circovirus-associated diseases. PCV2 replication could be promoted by low doses of ochratoxin A (OTA) as in our previous study and selenium has been shown to attenuate PCV2 replication. However, the underlying mechanism remains unclear. The aim of the study was to investigate the effects of selenomethionine (SeMet), the major component of organic selenium, on OTA-induced PCV2 replication promotion and its potential mechanism. The present study demonstrates that OTA could promote PCV2 replication as measured by cap protein expression, viral titer, viral DNA copies and the number of infected cells. In addition, OTA could activate autophagy as indicated by up-regulated light chain 3 (LC3)-II and autophagy-related protein 5 expressions and autophagosome formation. Further, OTA could down-regulate p-AKT and p-mTOR expressions and OTA-induced autophagy was inhibited when insulin was applied. SeMet at 2, 4 and 6 μM had significant inhibiting effects against OTA-induced PCV2 replication promotion. Furthermore, SeMet could attenuate OTA-induced autophagy and up-regulate OTA-induced p-AKT and p-mTOR expression inhibition. Rapamycin, an inhibitor of AKT/mTOR, could reverse the effects of SeMet on OTA-induced autophagy and the PCV2 replication promotion. In conclusion, SeMet could block OTA-induced PCV2 replication promotion by inhibiting autophagy by activating the AKT/mTOR pathway. Therefore, SeMet supplementation could be an effective prophylactic strategy against PCV2 infections and autophagy may be a potential marker to develop novel anti-PCV2 drugs.
Figures





References
-
- Todd D, Bendinelli M, Biagini P, Hino S, Mankertz A, Mishiro S, Niel C, Okamoto H, Raidal S, Ritchie BW, Teo GC (2005) Virus taxonomy, 8th report of the International Committee for the taxonomy of viruses. pp 327–334
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

