Data sharing and reanalysis of randomized controlled trials in leading biomedical journals with a full data sharing policy: survey of studies published in The BMJ and PLOS Medicine
- PMID: 29440066
- PMCID: PMC5809812
- DOI: 10.1136/bmj.k400
Data sharing and reanalysis of randomized controlled trials in leading biomedical journals with a full data sharing policy: survey of studies published in The BMJ and PLOS Medicine
Abstract
Objectives: To explore the effectiveness of data sharing by randomized controlled trials (RCTs) in journals with a full data sharing policy and to describe potential difficulties encountered in the process of performing reanalyses of the primary outcomes.
Design: Survey of published RCTs.
Setting: PubMed/Medline.
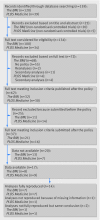
Eligibility criteria: RCTs that had been submitted and published by The BMJ and PLOS Medicine subsequent to the adoption of data sharing policies by these journals.
Main outcome measure: The primary outcome was data availability, defined as the eventual receipt of complete data with clear labelling. Primary outcomes were reanalyzed to assess to what extent studies were reproduced. Difficulties encountered were described.
Results: 37 RCTs (21 from The BMJ and 16 from PLOS Medicine) published between 2013 and 2016 met the eligibility criteria. 17/37 (46%, 95% confidence interval 30% to 62%) satisfied the definition of data availability and 14 of the 17 (82%, 59% to 94%) were fully reproduced on all their primary outcomes. Of the remaining RCTs, errors were identified in two but reached similar conclusions and one paper did not provide enough information in the Methods section to reproduce the analyses. Difficulties identified included problems in contacting corresponding authors and lack of resources on their behalf in preparing the datasets. In addition, there was a range of different data sharing practices across study groups.
Conclusions: Data availability was not optimal in two journals with a strong policy for data sharing. When investigators shared data, most reanalyses largely reproduced the original results. Data sharing practices need to become more widespread and streamlined to allow meaningful reanalyses and reuse of data.
Trial registration: Open Science Framework osf.io/c4zke.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare that (1) No authors have support from any company for the submitted work; (2) FN has relationships (travel/accommodations expenses covered/reimbursed) with Servier, BMS, Lundbeck, and Janssen who might have an interest in the work submitted in the previous three years. In the past three years PJ received a fellowship/grant from GSK for her PhD as part of a public-private collaboration. CS, IC, DF, DM, and JPAI have no relationship with any company that might have an interest in the work submitted; (3) no author’s spouse, partner, or children have any financial relationships that could be relevant to the submitted work; and (4) none of the authors has any non-financial interests that could be relevant to the submitted work.
Figures
Comment in
-
Data sharing in medical research.BMJ. 2018 Feb 14;360:k510. doi: 10.1136/bmj.k510. BMJ. 2018. PMID: 29444885 No abstract available.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous