Role of eIF2α Kinases in Translational Control and Adaptation to Cellular Stress
- PMID: 29440070
- PMCID: PMC6028073
- DOI: 10.1101/cshperspect.a032870
Role of eIF2α Kinases in Translational Control and Adaptation to Cellular Stress
Abstract
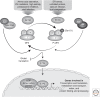
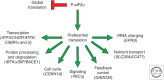
A central mechanism regulating translation initiation in response to environmental stress involves phosphorylation of the α subunit of eukaryotic initiation factor 2 (eIF2α). Phosphorylation of eIF2α causes inhibition of global translation, which conserves energy and facilitates reprogramming of gene expression and signaling pathways that help to restore protein homeostasis. Coincident with repression of protein synthesis, many gene transcripts involved in the stress response are not affected or are even preferentially translated in response to increased eIF2α phosphorylation by mechanisms involving upstream open reading frames (uORFs). This review highlights the mechanisms regulating eIF2α kinases, the role that uORFs play in translational control, and the impact that alteration of eIF2α phosphorylation by gene mutations or small molecule inhibitors can have on health and disease.
Copyright © 2018 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures


References
-
- Axten JM, Medina JR, Feng Y, Shu A, Romeril SP, Grant SW, Li WH, Heerding DA, Minthorn E, Mencken T, et al. 2012. Discovery of 7-methyl-5-(1-{[3-(trifluoromethyl)phenyl]acetyl}-2,3-dihydro-1H-indol-5-yl)-7H-p yrrolo[2,3-d]pyrimidin-4-amine (GSK2606414), a potent and selective first-in-class inhibitor of protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK). J Med Chem 55: 7193–7207. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
