Adaptive design clinical trials: a review of the literature and ClinicalTrials.gov
- PMID: 29440155
- PMCID: PMC5829673
- DOI: 10.1136/bmjopen-2017-018320
Adaptive design clinical trials: a review of the literature and ClinicalTrials.gov
Abstract
Objectives: This review investigates characteristics of implemented adaptive design clinical trials and provides examples of regulatory experience with such trials.
Design: Review of adaptive design clinical trials in EMBASE, PubMed, Cochrane Registry of Controlled Clinical Trials, Web of Science and ClinicalTrials.gov. Phase I and seamless Phase I/II trials were excluded. Variables extracted from trials included basic study characteristics, adaptive design features, size and use of independent data monitoring committees (DMCs) and blinded interim analyses. We also examined use of the adaptive trials in new drug submissions to the Food and Drug Administration (FDA) and European Medicines Agency (EMA) and recorded regulators' experiences with adaptive designs.
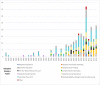
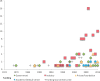
Results: 142 studies met inclusion criteria. There has been a recent growth in publicly reported use of adaptive designs among researchers around the world. The most frequently appearing types of adaptations were seamless Phase II/III (57%), group sequential (21%), biomarker adaptive (20%), and adaptive dose-finding designs (16%). About one-third (32%) of trials reported an independent DMC, while 6% reported blinded interim analysis. We found that 9% of adaptive trials were used for FDA product approval consideration, and 12% were used for EMA product approval consideration. International regulators had mixed experiences with adaptive trials. Many product applications with adaptive trials had extensive correspondence between drug sponsors and regulators regarding the adaptive designs, in some cases with regulators requiring revisions or alterations to research designs.
Conclusions: Wider use of adaptive designs will necessitate new drug application sponsors to engage with regulatory scientists during planning and conduct of the trials. Investigators need to more consistently report protections intended to preserve confidentiality and minimise potential operational bias during interim analysis.
Keywords: EMA; FDA; adaptive design; clinical trial; data monitoring committee; flexible design; history; interim analysis; policy; regulation; review.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: ASK had financial support from the Laura and John Arnold Foundation and Harvard Program in Therapeutic Science and LEB had support from Brigham and Women’s Hospital Innovation Hub for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Figures

Comment in
-
Global COVID-19 vaccine roll-out: time to randomise vaccine allocation?Lancet. 2021 May 15;397(10287):1804-1805. doi: 10.1016/S0140-6736(21)00895-3. Lancet. 2021. PMID: 33992138 Free PMC article. No abstract available.
References
-
- United States Government. Rules committee print 114-67, text of House amendment to the Senate, amendment to H.R. 34, Tsunami Warning, Education, and Research Act of 2015, 2016:162–3. 114th Congress http://docs.house.gov/billsthisweek/20161128/CPRT-114-HPRT-RU00-SAHR34.pdf
-
- Hoel DG, Sobel M, Weiss GH. A survey of adaptive sampling for clinical trials : Elashoff RM, Perspectives in Biometrics. New York: Academic Press, 1975:30.
-
- U.S. Food and Drug Administration Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research. Guidance for industry: adaptive design clinical trials for drugs and biologics (Draft Guidance). MD: Silver Spring, 2010.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
