A Model Linking Sickle Cell Hemoglobinopathies and SMARCB1 Loss in Renal Medullary Carcinoma
- PMID: 29440190
- PMCID: PMC12283083
- DOI: 10.1158/1078-0432.CCR-17-3296
A Model Linking Sickle Cell Hemoglobinopathies and SMARCB1 Loss in Renal Medullary Carcinoma
Abstract
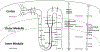
Renal medullary carcinoma (RMC) is a highly aggressive malignancy that predominantly afflicts young adults and adolescents with sickle hemoglobinopathies. It is characterized by complete loss of expression of the chromatin remodeler and tumor suppressor SMARCB1 Despite therapy, the outcomes of patients with RMC remain very poor, highlighting the need to understand the etiology of this cancer, and develop new diagnostic, preventive, and therapeutic strategies. A key knowledge gap in RMC biology is why sickle hemoglobinopathies predispose to the development of this cancer. We propose a model wherein the extreme conditions of hypoxia and hypertonicity of the renal medulla, combined with regional ischemia induced by red blood cell sickling, activate DNA repair mechanisms to drive deletions and translocations in SMARCB1, which is localized in a fragile region of chromosome 22. This mechanism would explain the linkage between RMC and sickle hemoglobinopathies, as well as the age dependence and predilection of RMC toward the right kidney.Significance: This perspective proposes an integrated and testable model of renal medullary carcinoma pathogenesis. Insights provided by this model can additionally inform other malignancies arising from the renal medulla and/or associated with loss of the SMARCB1 tumor suppressor gene. Clin Cancer Res; 24(9); 2044-9. ©2018 AACR.
©2018 American Association for Cancer Research.
Conflict of interest statement
Figures

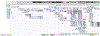
References
-
- Davis CJ Jr., Mostofi FK, Sesterhenn IA. Renal medullary carcinoma. The seventh sickle cell nephropathy. The American journal of surgical pathology 1995;19:1–11. - PubMed
-
- Alvarez O, Rodriguez MM, Jordan L, Sarnaik S. Renal medullary carcinoma and sickle cell trait: A systematic review. Pediatr Blood Cancer 2015;62:1694–9. - PubMed
-
- Hollmann TJ, Hornick JL. INI1-deficient tumors: diagnostic features and molecular genetics. The American journal of surgical pathology 2011;35:e47–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

