Calcitonin receptors are ancient modulators for rhythms of preferential temperature in insects and body temperature in mammals
- PMID: 29440246
- PMCID: PMC5830927
- DOI: 10.1101/gad.307884.117
Calcitonin receptors are ancient modulators for rhythms of preferential temperature in insects and body temperature in mammals
Abstract
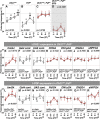
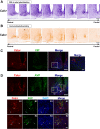
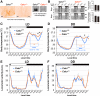
Daily body temperature rhythm (BTR) is essential for maintaining homeostasis. BTR is regulated separately from locomotor activity rhythms, but its molecular basis is largely unknown. While mammals internally regulate BTR, ectotherms, including Drosophila, exhibit temperature preference rhythm (TPR) behavior to regulate BTR. Here, we demonstrate that the diuretic hormone 31 receptor (DH31R) mediates TPR during the active phase in Drosophila DH31R is expressed in clock cells, and its ligand, DH31, acts on clock cells to regulate TPR during the active phase. Surprisingly, the mouse homolog of DH31R, calcitonin receptor (Calcr), is expressed in the suprachiasmatic nucleus (SCN) and mediates body temperature fluctuations during the active phase in mice. Importantly, DH31R and Calcr are not required for coordinating locomotor activity rhythms. Our results represent the first molecular evidence that BTR is regulated distinctly from locomotor activity rhythms and show that DH31R/Calcr is an ancient specific mediator of BTR during the active phase in organisms ranging from ectotherms to endotherms.
Keywords: Calcr; DH31R; body temperature rhythm; calcitonin receptor; circadian rhythm; temperature preference rhythm; thermoregulation.
© 2018 Goda et al.; Published by Cold Spring Harbor Laboratory Press.
Figures



Similar articles
-
Drosophila Temperature Preference Rhythms: An Innovative Model to Understand Body Temperature Rhythms.Int J Mol Sci. 2019 Apr 23;20(8):1988. doi: 10.3390/ijms20081988. Int J Mol Sci. 2019. PMID: 31018551 Free PMC article. Review.
-
Molecular and Neural Mechanisms of Temperature Preference Rhythm in Drosophila melanogaster.J Biol Rhythms. 2023 Aug;38(4):326-340. doi: 10.1177/07487304231171624. Epub 2023 May 24. J Biol Rhythms. 2023. PMID: 37222551 Free PMC article. Review.
-
Drosophila DH31 Neuropeptide and PDF Receptor Regulate Night-Onset Temperature Preference.J Neurosci. 2016 Nov 16;36(46):11739-11754. doi: 10.1523/JNEUROSCI.0964-16.2016. J Neurosci. 2016. PMID: 27852781 Free PMC article.
-
Circadian rhythm of temperature preference and its neural control in Drosophila.Curr Biol. 2012 Oct 9;22(19):1851-7. doi: 10.1016/j.cub.2012.08.006. Epub 2012 Sep 13. Curr Biol. 2012. PMID: 22981774 Free PMC article.
-
Neuropeptides PDF and DH31 hierarchically regulate free-running rhythmicity in Drosophila circadian locomotor activity.Sci Rep. 2019 Jan 29;9(1):838. doi: 10.1038/s41598-018-37107-3. Sci Rep. 2019. PMID: 30696873 Free PMC article.
Cited by
-
Calcitonin receptor antibody validation and expression in the rodent brain.Cephalalgia. 2022 Aug;42(9):815-826. doi: 10.1177/03331024221084029. Epub 2022 Apr 11. Cephalalgia. 2022. PMID: 35410497 Free PMC article.
-
Whole-genome resequencing reveals genetic diversity and adaptive evolution in Chinese honeybee (Apis cerana cerana) in Guizhou, China.Front Genet. 2024 May 17;15:1352455. doi: 10.3389/fgene.2024.1352455. eCollection 2024. Front Genet. 2024. PMID: 38826805 Free PMC article.
-
CGRP physiology, pharmacology, and therapeutic targets: migraine and beyond.Physiol Rev. 2023 Apr 1;103(2):1565-1644. doi: 10.1152/physrev.00059.2021. Epub 2022 Dec 1. Physiol Rev. 2023. PMID: 36454715 Free PMC article. Review.
-
A Symphony of Signals: Intercellular and Intracellular Signaling Mechanisms Underlying Circadian Timekeeping in Mice and Flies.Int J Mol Sci. 2019 May 13;20(9):2363. doi: 10.3390/ijms20092363. Int J Mol Sci. 2019. PMID: 31086044 Free PMC article. Review.
-
G-Protein Coupled Receptors (GPCRs) in Insects-A Potential Target for New Insecticide Development.Molecules. 2021 May 18;26(10):2993. doi: 10.3390/molecules26102993. Molecules. 2021. PMID: 34069969 Free PMC article. Review.
References
-
- Abrahamson EE, Moore RY. 2001. Suprachiasmatic nucleus in the mouse: retinal innervation, intrinsic organization and efferent projections. Brain Res 916: 172–191. - PubMed
-
- Aschoff J. 1983. Circadian control of body temperature. J Therm Biol 8: 143–147.
-
- Barsh GS, Schwartz MW. 2002. Genetic approaches to studying energy balance: perception and integration. Nat Rev Genet 3: 589–600. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
