Essential immunologic orchestrators of intestinal homeostasis
- PMID: 29440266
- PMCID: PMC6352895
- DOI: 10.1126/sciimmunol.aao1605
Essential immunologic orchestrators of intestinal homeostasis
Abstract
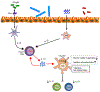
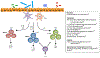
Over the past 25 years, substantial advances have been made in our understanding of the cellular and molecular pathways that are essential to maintain a state of health in the mammalian gastrointestinal tract, an organ that is densely colonized by both immune cells and trillions of microbes. Seminal studies in the 1990s identified that several cytokines, antigen-presentation molecules, and components of the T cell receptor were necessary to prevent the development of spontaneous intestinal inflammation in mice. Subsequent research revealed that these pathways orchestrate beneficial interactions with intestinal microbes, involve complex communication between innate and adaptive immune cells, and can be dysregulated in human inflammatory bowel disease. Here, we discuss how these early findings set the stage for numerous other advances and shaped our current knowledge of host-microbiota interactions and intestinal homeostasis in mammals. It is expected that continued investigation of these areas will define previously unknown immunologic mechanisms of tolerance and inflammation in the intestine that can be exploited to benefit human health.
Copyright © 2018 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures


References
-
- Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W, Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75, 263 (October 22, 1993). - PubMed
-
- Mombaerts P et al., Spontaneous development of inflammatory bowel disease in T cell receptor mutant mice. Cell 75, 274 (October 22, 1993). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

