Targeting the MAPK Pathway in RAS Mutant Cancers
- PMID: 29440321
- PMCID: PMC6211377
- DOI: 10.1101/cshperspect.a031492
Targeting the MAPK Pathway in RAS Mutant Cancers
Abstract
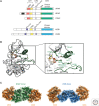
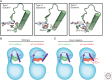
Despite decades of extensive drug discovery efforts, there are currently no targeted therapies approved to treat KRAS mutant cancers. In this review, we highlight the challenges and opportunities in targeting KRAS mutant tumors through inhibition of mitogen-activated protein kinase (MAPK) signaling with conformation-specific kinase inhibitors. Through structural analysis and mechanistic studies with BRAF and mitogen-activated protein kinase (MEK) inhibitors, we describe how kinase-dependent and -independent functions of MAPK signaling components regulate KRAS-driven tumorigenesis and how these insights can be used to treat RAS mutant cancers with small molecule kinase inhibitors.
Copyright © 2018 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures


References
-
- Anforth R, Tembe V, Blumetti T, Fernandez-Penas P. 2012a. Mutational analysis of cutaneous squamous cell carcinomas and verrucal keratosis in patients taking BRAF inhibitors. Pigment Cell Melanoma Res 25: 569–572. - PubMed
-
- Anforth RM, Blumetti TC, Kefford RF, Sharma R, Scolyer RA, Kossard S, Long GV, Fernandez-Penas P. 2012b. Cutaneous manifestations of dabrafenib (GSK2118436): A selective inhibitor of mutant BRAF in patients with metastatic melanoma. Br J Dermatol 167: 1153–1160. - PubMed
-
- Blake JF, Burkard M, Chan J, Chen H, Chou KJ, Diaz D, Dudley DA, Gaudino JJ, Gould SE, Grina J, et al. 2016. Discovery of (S)-1-(1-(4-Chloro-3-fluorophenyl)-2-hydroxyethyl)-4-(2-((1-methyl-1H-pyrazol-5-yl)amino)pyrimidin-4-yl)pyridin-2(1H)-one (GDC-0994), an extracellular signal-regulated kinase 1/2 (ERK1/2) inhibitor in early clinical development. J Med Chem 59: 5650–5660. - PubMed
-
- Bollag G, Tsai J, Zhang J, Zhang C, Ibrahim P, Nolop K, Hirth P. 2012. Vemurafenib: The first drug approved for BRAF-mutant cancer. Nat Rev Drug Discov 11: 873–886. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
