Natural History, Clinical Manifestations, and Pathogenesis of Hepatitis A
- PMID: 29440324
- PMCID: PMC6120688
- DOI: 10.1101/cshperspect.a031708
Natural History, Clinical Manifestations, and Pathogenesis of Hepatitis A
Abstract
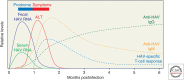
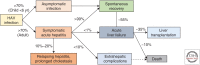
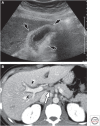
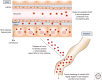
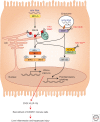
Hepatitis A virus (HAV) is transmitted by the fecal-oral route and is a major cause of acute viral hepatitis. The clinical manifestations of HAV infection range from asymptomatic infection to acute liver failure (ALF), but do not include progression to chronic hepatitis. Risk factors for severe acute hepatitis A are older age (>40 years) and preexisting liver disease. Some patients may show atypical clinical features such as relapsing hepatitis, prolonged cholestasis, or extrahepatic manifestations. Almost all hepatitis A patients spontaneously recover with supportive care. However, in the case of ALF (<1%), intensive care and urgent decision on liver transplantation are required. Liver injury during hepatitis A is not directly caused by HAV but is known to be caused by immune-mediated mechanisms. In this review, the natural history and clinical manifestations of hepatitis A are described. In addition, mechanisms of immunopathogenesis in hepatitis A are discussed.
Copyright © 2018 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures
References
-
- Amado Leon LA, de Almeida AJ, de Paula VS, Tourinho RS, Villela DA, Gaspar AM, Lewis-Ximenez LL, Pinto MA. 2015. Longitudinal study of hepatitis A infection by saliva sampling: The kinetics of HAV markers in saliva revealed the application of saliva tests for hepatitis A study. PLoS ONE 10: e0145454. - PMC - PubMed
-
- Armstrong GL, Bell BP. 2002. Hepatitis A virus infections in the United States: Model-based estimates and implications for childhood immunization. Pediatrics 109: 839–845. - PubMed
-
- Asher LV, Binn LN, Mensing TL, Marchwicki RH, Vassell RA, Young GD. 1995. Pathogenesis of hepatitis A in orally inoculated owl monkeys (Aotus trivirgatus). J Med Virol 47: 260–268. - PubMed
-
- Chen CM, Chen SC, Yang HY, Yang ST, Wang CM. 2016. Hospitalization and mortality due to hepatitis A in Taiwan: A 15-year nationwide cohort study. J Viral Hepat 23: 940–945. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
