Fetal and Maternal Innate Immunity Receptors Have Opposing Effects on the Severity of Experimental Malaria in Pregnancy: Beneficial Roles for Fetus-Derived Toll-Like Receptor 4 and Type I Interferon Receptor 1
- PMID: 29440369
- PMCID: PMC5913849
- DOI: 10.1128/IAI.00708-17
Fetal and Maternal Innate Immunity Receptors Have Opposing Effects on the Severity of Experimental Malaria in Pregnancy: Beneficial Roles for Fetus-Derived Toll-Like Receptor 4 and Type I Interferon Receptor 1
Abstract
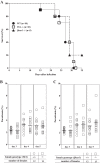
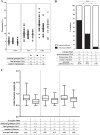
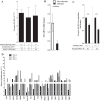
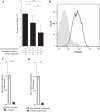
Malaria in pregnancy (MiP) is a distinctive clinical form of Plasmodium infection and is a cause of placental insufficiency leading to poor pregnancy outcomes. Maternal innate immunity responses play a decisive role in the development of placental inflammation, but the action of fetus-derived factors in MiP outcomes has been overlooked. We investigated the role of the Tlr4 and Ifnar1 genes, taking advantage of heterogenic mating strategies to dissect the effects mediated by maternally and fetally derived Toll-like receptor 4 (TLR4) or type I interferon receptor 1 (IFNAR1). Using a mouse infection system displaying severe MiP outcomes, we found that the expressions of TLR4 and IFNAR1 in the maternal compartment take part in deleterious MiP outcomes, but their fetal counterparts patently counteract these effects. We uncovered that fetal TLR4 contributes to the in vitro uptake of infected erythrocytes by trophoblasts and to the innate immune response in the placenta, offering robust protection of fetus viability, but had no sensible impact on the placental parasite burden. In contrast, we observed that the expression of IFNAR1 in the fetal compartment was associated with a reduced placental parasite burden but had little beneficial effect on fetus outcomes. Furthermore, the downregulation of Ifnar1 expression in infected placentas and in trophoblasts exposed to infected erythrocytes indicated that the interferon-IFNAR1 pathway is involved in the trophoblast response to infection. This work unravels that maternal and fetal counterparts of innate immune pathways drive opposing responses in murine placental malaria and implicates the activation of innate receptors in fetal trophoblast cells in the control of placental infection and in the protection of the fetus.
Keywords: IFNAR1; TLR4; fetal innate immunity; pregnancy malaria.
Copyright © 2018 Rodrigues-Duarte et al.
Figures

Similar articles
-
Insight Into TLR4-Mediated Immunomodulation in Normal Pregnancy and Related Disorders.Front Immunol. 2020 May 19;11:807. doi: 10.3389/fimmu.2020.00807. eCollection 2020. Front Immunol. 2020. PMID: 32508811 Free PMC article. Review.
-
Maternal-Fetal Conflict During Infection: Lessons From a Mouse Model of Placental Malaria.Front Microbiol. 2019 May 24;10:1126. doi: 10.3389/fmicb.2019.01126. eCollection 2019. Front Microbiol. 2019. PMID: 31178840 Free PMC article.
-
TLR4-Endothelin Axis Controls Syncytiotrophoblast Motility and Confers Fetal Protection in Placental Malaria.Infect Immun. 2021 Jul 15;89(8):e0080920. doi: 10.1128/IAI.00809-20. Epub 2021 Jul 15. Infect Immun. 2021. PMID: 34061587 Free PMC article.
-
TLR4-Mediated Placental Pathology and Pregnancy Outcome in Experimental Malaria.Sci Rep. 2017 Aug 17;7(1):8623. doi: 10.1038/s41598-017-08299-x. Sci Rep. 2017. PMID: 28819109 Free PMC article.
-
Toll-like receptors and their role in the trophoblast.Placenta. 2005 Aug;26(7):540-7. doi: 10.1016/j.placenta.2004.08.010. Placenta. 2005. PMID: 15993703 Review.
Cited by
-
Mouse fetal growth restriction through parental and fetal immune gene variation and intercellular communications cascade.Nat Commun. 2022 Jul 29;13(1):4398. doi: 10.1038/s41467-022-32171-w. Nat Commun. 2022. PMID: 35906236 Free PMC article.
-
Regulation of inflammation during gestation and birth outcomes: Inflammatory cytokine balance predicts birth weight and length.Am J Hum Biol. 2019 May;31(3):e23245. doi: 10.1002/ajhb.23245. Epub 2019 Apr 13. Am J Hum Biol. 2019. PMID: 30980448 Free PMC article.
-
Insight Into TLR4-Mediated Immunomodulation in Normal Pregnancy and Related Disorders.Front Immunol. 2020 May 19;11:807. doi: 10.3389/fimmu.2020.00807. eCollection 2020. Front Immunol. 2020. PMID: 32508811 Free PMC article. Review.
-
The vertical transmission of Salmonella Enteritidis in a One-Health context.One Health. 2022 Dec 5;16:100469. doi: 10.1016/j.onehlt.2022.100469. eCollection 2023 Jun. One Health. 2022. PMID: 36507074 Free PMC article. Review.
-
Maternal-Fetal Conflict During Infection: Lessons From a Mouse Model of Placental Malaria.Front Microbiol. 2019 May 24;10:1126. doi: 10.3389/fmicb.2019.01126. eCollection 2019. Front Microbiol. 2019. PMID: 31178840 Free PMC article.
References
-
- Maitra N, Joshi M, Hazra M. 1993. Maternal manifestations of malaria in pregnancy: a review. Indian J Matern Child Health 4:98–101. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

