Large Visual Stimuli Induce Two Distinct Gamma Oscillations in Primate Visual Cortex
- PMID: 29440388
- PMCID: PMC5852657
- DOI: 10.1523/JNEUROSCI.2270-17.2017
Large Visual Stimuli Induce Two Distinct Gamma Oscillations in Primate Visual Cortex
Abstract
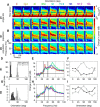
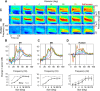
Recent studies have shown the existence of two gamma rhythms in the hippocampus subserving different functions but, to date, primate studies in primary visual cortex have reported a single gamma rhythm. Here, we show that large visual stimuli induce a slow gamma (25-45 Hz) in area V1 of two awake adult female bonnet monkeys and in the EEG of 15 human subjects (7 males and 8 females), in addition to the traditionally known fast gamma (45-70 Hz). The two rhythms had different tuning characteristics for stimulus orientation, contrast, drift speed, and size. Further, fast gamma had short latency, strongly entrained spikes and was coherent over short distances, reflecting short-range processing, whereas slow gamma appeared to reflect long-range processing. Together, two gamma rhythms can potentially provide better coding or communication mechanisms and a more comprehensive biomarker for diagnosis of mental disorders.SIGNIFICANCE STATEMENT Gamma rhythm has been associated with high-level cognitive functions such as attention and feature binding and has been reported to be abnormal in brain disorders such as autism and schizophrenia. Unlike previous studies that have shown a single gamma rhythm in the primate visual cortex, we found that large visual gratings induce two distinct gamma oscillations in both monkey LFP and human EEG. These rhythms, termed slow (25-45 Hz) and fast (45-70 Hz), exhibited distinct tuning preferences, latencies, and coherence profiles, potentially reflecting processing at two different ranges. Multiple gamma oscillations in visual cortex may provide a richer representation of external visual stimuli and could be used for developing brain-machine interfacing applications and screening tests for neuropsychiatric disorders.
Keywords: EEG; LFP; area V1; gamma; oscillation; rhythm.
Copyright © 2018 Murty et al.
Figures






References
-
- Berens P. (2009) CircStat: A MATLAB toolbox for circular statistics. Journal of Statistical Software. Available at: https://www.jstatsoft.org/article/view/v031i10. Accessed 5 November 2017.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
