Chimeric antigen receptor T cells form nonclassical and potent immune synapses driving rapid cytotoxicity
- PMID: 29440406
- PMCID: PMC5834689
- DOI: 10.1073/pnas.1716266115
Chimeric antigen receptor T cells form nonclassical and potent immune synapses driving rapid cytotoxicity
Erratum in
-
Correction for Davenport et al., Chimeric antigen receptor T cells form nonclassical and potent immune synapses driving rapid cytotoxicity.Proc Natl Acad Sci U S A. 2019 May 28;116(22):11075-11076. doi: 10.1073/pnas.1907056116. Epub 2019 May 20. Proc Natl Acad Sci U S A. 2019. PMID: 31109999 Free PMC article. No abstract available.
Abstract
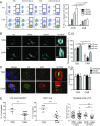
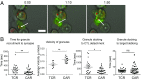
Chimeric antigen receptor T (CAR-T) cells are effective serial killers with a faster off-rate from dying tumor cells than CAR-T cells binding target cells through their T cell receptor (TCR). Here we explored the functional consequences of CAR-mediated signaling using a dual-specific CAR-T cell, where the same cell was triggered via TCR (tcrCTL) or CAR (carCTL). The carCTL immune synapse lacked distinct LFA-1 adhesion rings and was less reliant on LFA to form stable conjugates with target cells. carCTL receptors associated with the synapse were found to be disrupted and formed a convoluted multifocal pattern of Lck microclusters. Both proximal and distal receptor signaling pathways were induced more rapidly and subsequently decreased more rapidly in carCTL than in tcrCTL. The functional consequence of this rapid signaling in carCTL cells included faster lytic granule recruitment to the immune synapse, correlating with faster detachment of the CTL from the target cell. This study provides a mechanism for how CAR-T cells can debulk large tumor burden quickly and may contribute to further refinement of CAR design for enhancing the quality of signaling and programming of the T cell.
Keywords: cell death; chimeric antigen receptor; cytotoxic T lymphocyte; cytotoxicity; immune synapse.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

