Intensity and duration of TCR signaling is limited by p38 phosphorylation of ZAP-70T293 and destabilization of the signalosome
- PMID: 29440413
- PMCID: PMC5834678
- DOI: 10.1073/pnas.1713301115
Intensity and duration of TCR signaling is limited by p38 phosphorylation of ZAP-70T293 and destabilization of the signalosome
Abstract
ZAP-70 is a tyrosine kinase that is essential for initiation of T cell antigen receptor (TCR) signaling. We have found that T cell p38 MAP kinase (MAPK), which is directly phosphorylated and activated by ZAP-70 downstream of the TCR, in turn phosphorylates Thr-293 in the interdomain B region of ZAP-70. Mutant T cells expressing ZAP-70 with an alanine substitution at this residue (ZAP-70T293A) had enhanced TCR proximal signaling and increased effector responses. Lack of ZAP-70T293 phosphorylation increased association of ZAP-70 with the TCR and prolonged the existence of TCR signaling microclusters. These results identify a tight negative feedback loop in which ZAP-70-activated p38 reciprocally phosphorylates ZAP-70 and destabilizes the signaling complex.
Keywords: MAP kinase; T cell antigen receptor; immune synapse; signal transduction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

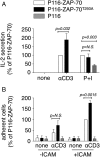


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

