Epitope-specific monoclonal antibodies to FSHβ increase bone mass
- PMID: 29440419
- PMCID: PMC5834707
- DOI: 10.1073/pnas.1718144115
Epitope-specific monoclonal antibodies to FSHβ increase bone mass
Abstract
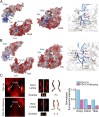
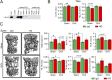
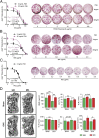
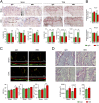
Pituitary hormones have long been thought solely to regulate single targets. Challenging this paradigm, we discovered that both anterior and posterior pituitary hormones, including FSH, had other functions in physiology. We have shown that FSH regulates skeletal integrity, and, more recently, find that FSH inhibition reduces body fat and induces thermogenic adipose tissue. A polyclonal antibody raised against a short, receptor-binding epitope of FSHβ was found not only to rescue bone loss postovariectomy, but also to display marked antiobesity and probeiging actions. Questioning whether a single agent could be used to treat two medical conditions of public health importance--osteoporosis and obesity--we developed two further monoclonal antibodies, Hf2 and Mf4, against computationally defined receptor-binding epitopes of FSHβ. Hf2 has already been shown to reduce body weight and fat mass and cause beiging in mice on a high-fat diet. Here, we show that Hf2, which binds mouse Fsh in immunoprecipitation assays, also increases cortical thickness and trabecular bone volume, and microstructural parameters, in sham-operated and ovariectomized mice, noted on microcomputed tomography. This effect was largely recapitulated with Mf4, which inhibited bone resorption by osteoclasts and stimulated new bone formation by osteoblasts. These effects were exerted in the absence of alterations in serum estrogen in wild-type mice. We also reconfirm the existence of Fshrs in bone by documenting the specific binding of fluorescently labeled FSH, FSH-CH, in vivo. Our study provides the framework for the future development of an FSH-based therapeutic that could potentially target both bone and fat.
Keywords: FSH monoclonal antibody; FSH polyclonal antibody; FSH receptor; antiobesity; osteoporosis treatment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Greendale GA, Sowers M. The menopause transition. Endocrinol Metab Clin North Am. 1997;26:261–277. - PubMed
-
- Sowers MR, et al. Endogenous hormones and bone turnover markers in pre- and perimenopausal women: SWAN. Osteoporos Int. 2003;14:191–197. - PubMed
-
- Randolph JF, Jr, et al. The value of follicle-stimulating hormone concentration and clinical findings as markers of the late menopausal transition. J Clin Endocrinol Metab. 2006;91:3034–3040. - PubMed
-
- Sowers M, Pope S, Welch G, Sternfeld B, Albrecht G. The association of menopause and physical functioning in women at midlife. J Am Geriatr Soc. 2001;49:1485–1492. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

