Altered Signaling in the Descending Pain-modulatory System after Short-Term Infusion of the μ-Opioid Agonist Remifentanil
- PMID: 29440535
- PMCID: PMC6705904
- DOI: 10.1523/JNEUROSCI.2496-17.2018
Altered Signaling in the Descending Pain-modulatory System after Short-Term Infusion of the μ-Opioid Agonist Remifentanil
Abstract
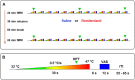
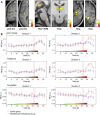
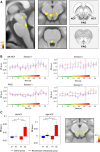
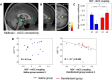
μ-Opioid receptor agonists are widely used within the contemporary treatment of pain, but abrupt opioid suspension, even after short-term infusion, can paradoxically increase the sensitivity to noxious stimuli, a phenomenon that has been, for example, reported after application of the fast-acting μ-opioid receptor agonist remifentanil. To investigate the mechanisms underlying the effects of discontinuation of remifentanil application on pain processing in the human CNS, we analyzed neuronal responses to thermal stimuli before and after a short-term infusion of remifentanil (30 min 0.1 μg/kg body weight/min) compared with control in the brain, brainstem, and spinal cord in drug-naive male volunteers using fMRI. Subsequent to remifentanil suspension, we observed reduced heat pain thresholds and increased neuronal responses in pain-encoding as well as in key regions of the descending pain-modulatory system, such as the periaqueductal gray matter, the nucleus cuneiformis, and the rostral ventromedial medulla. Moreover, the spinal pain-related multivoxel activity pattern showed an opioid-specific change after drug suspension. Importantly, remifentanil suspension increased the functional coupling between the nucleus cuneiformis and the rostral anterior cingulate cortex, and the coupling strength between the rostral anterior cingulate cortex and the nucleus cuneiformis correlated negatively with the individual pain threshold after opioid suspension. These findings demonstrate that, already subsequent to a short-term infusion of the μ-opioid receptor agonist remifentanil, signaling in the descending pain-modulatory system is fundamentally altered and that these changes are directly related to the behavioral sensitivity to pain.SIGNIFICANCE STATEMENT Opioids are widely used in modern medicine, but, in addition to their known side effects, it is increasingly recognized that opioids can also increase sensitivity to pain subsequent to their use. Using the fast-acting μ-opioid receptor agonist remifentanil and fMRI in healthy male volunteers, this study demonstrates how signaling changes occur along the entire descending pain-modulatory pathway after opioid discontinuation and how these alterations are closely linked to increased behavioral pain sensitivity. Particularly by revealing modified responses in pain-modulatory brainstem regions that have been previously demonstrated to be causally involved in acute opioid withdrawal effects in rodents, the data provide a plausible neuronal mechanism by which the increased sensitivity to pain after opioid suspension is mediated in humans.
Keywords: fMRI; opioids; pain; remifentanil.
Copyright © 2018 the authors 0270-6474/18/382454-17$15.00/0.
Figures


Similar articles
-
Imaging human cerebral pain modulation by dose-dependent opioid analgesia: a positron emission tomography activation study using remifentanil.Anesthesiology. 2007 Mar;106(3):548-56. doi: 10.1097/00000542-200703000-00020. Anesthesiology. 2007. PMID: 17325514 Clinical Trial.
-
Short-term infusion of the mu-opioid agonist remifentanil in humans causes hyperalgesia during withdrawal.Pain. 2003 Nov;106(1-2):49-57. doi: 10.1016/s0304-3959(03)00276-8. Pain. 2003. PMID: 14581110 Clinical Trial.
-
The mu-opioid receptor agonist remifentanil induces acute dysphoria irrespective of its analgesic properties.J Psychopharmacol. 2010 Mar;24(3):355-61. doi: 10.1177/0269881108095811. Epub 2008 Sep 18. J Psychopharmacol. 2010. PMID: 18801832 Clinical Trial.
-
Remifentanil and other opioids.Handb Exp Pharmacol. 2008;(182):283-311. doi: 10.1007/978-3-540-74806-9_14. Handb Exp Pharmacol. 2008. PMID: 18175097 Review.
-
Remifentanil tolerance and hyperalgesia: short-term gain, long-term pain?Anaesthesia. 2016 Nov;71(11):1347-1362. doi: 10.1111/anae.13602. Anaesthesia. 2016. PMID: 27734470 Review.
Cited by
-
Neuroimaging of opioid effects in humans across conditions of acute administration, chronic pain therapy, and opioid use disorder.Trends Neurosci. 2024 Jun;47(6):418-431. doi: 10.1016/j.tins.2024.04.005. Epub 2024 May 17. Trends Neurosci. 2024. PMID: 38762362 Free PMC article. Review.
-
Reliability of task-based fMRI in the dorsal horn of the human spinal cord.bioRxiv [Preprint]. 2024 Jun 25:2023.12.22.572825. doi: 10.1101/2023.12.22.572825. bioRxiv. 2024. Update in: Imaging Neurosci (Camb). 2024 Aug 22;2:imag-2-00273. doi: 10.1162/imag_a_00273. PMID: 38187724 Free PMC article. Updated. Preprint.
-
Ultra-high-field imaging reveals increased whole brain connectivity underpins cognitive strategies that attenuate pain.Elife. 2020 Sep 2;9:e55028. doi: 10.7554/eLife.55028. Elife. 2020. PMID: 32876049 Free PMC article.
-
Brainstem Pain-Modulation Circuitry and Its Plasticity in Neuropathic Pain: Insights From Human Brain Imaging Investigations.Front Pain Res (Lausanne). 2021 Jul 30;2:705345. doi: 10.3389/fpain.2021.705345. eCollection 2021. Front Pain Res (Lausanne). 2021. PMID: 35295481 Free PMC article. Review.
-
Gradual withdrawal of remifentanil delays initial post-operative analgesic demand after thyroid surgery; double-blinded, randomized controlled trial.BMC Anesthesiol. 2019 Apr 25;19(1):60. doi: 10.1186/s12871-019-0731-9. BMC Anesthesiol. 2019. PMID: 31027480 Free PMC article. Clinical Trial.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
