Type 2 Innate Lymphocytes Actuate Immunity Against Tumours and Limit Cancer Metastasis
- PMID: 29440650
- PMCID: PMC5811448
- DOI: 10.1038/s41598-018-20608-6
Type 2 Innate Lymphocytes Actuate Immunity Against Tumours and Limit Cancer Metastasis
Abstract
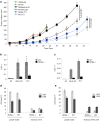
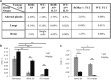
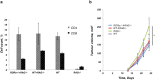
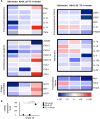
Type 2 innate lymphoid cells (ILC2) potentiate immune responses, however, their role in mediating adaptive immunity in cancer has not been assessed. Here, we report that mice genetically lacking ILC2s have significantly increased tumour growth rates and conspicuously higher frequency of circulating tumour cells (CTCs) and resulting metastasis to distal organs. Our data support the model that IL-33 dependent tumour-infiltrating ILC2s are mobilized from the lungs and other tissues through chemoattraction to enter tumours, and subsequently mediate tumour immune-surveillance by cooperating with dendritic cells to promote adaptive cytolytic T cell responses. We conclude that ILC2s play a fundamental, yet hitherto undescribed role in enhancing anti-cancer immunity and controlling tumour metastasis.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

