The distribution of blood eosinophil levels in a Japanese COPD clinical trial database and in the rest of the world
- PMID: 29440882
- PMCID: PMC5799851
- DOI: 10.2147/COPD.S144108
The distribution of blood eosinophil levels in a Japanese COPD clinical trial database and in the rest of the world
Abstract
Background: Blood eosinophil measurements may help to guide physicians on the use of inhaled corticosteroids (ICS) for patients with chronic obstructive pulmonary disease (COPD). Emerging data suggest that COPD patients with higher blood eosinophil counts may be at higher risk of exacerbations and more likely to benefit from combined ICS/long-acting beta2-agonist (LABA) treatment than therapy with a LABA alone. This analysis describes the distribution of blood eosinophil count at baseline in Japanese COPD patients in comparison with non-Japanese COPD patients.
Methods: A post hoc analysis of eosinophil distribution by percentage and absolute cell count was performed across 12 Phase II-IV COPD clinical studies (seven Japanese studies [N=848 available absolute eosinophil counts] and five global studies [N=5,397 available eosinophil counts] that included 246 Japanese patients resident in Japan with available counts). Blood eosinophil distributions were assessed at baseline, before blinded treatment assignment.
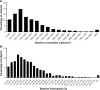
Findings: Among Japanese patients, the median (interquartile range) absolute eosinophil count was 170 cells/mm3 (100-280 cells/mm3). Overall, 612/1,094 Japanese patients (56%) had an absolute eosinophil count ≥150 cells/mm3 and 902/1,304 Japanese patients (69%) had a percentage eosinophil ≥2%. Among non-Japanese patients, these values were 160 (100-250) cells/mm3, 2,842/5,151 patients (55%), and 2,937/5,155 patients (57%), respectively. The eosinophil distribution among Japanese patients was similar to that among non-Japanese patients. Within multi-country studies with similar inclusion criteria, the eosinophil count was numerically lower in Japanese compared with non-Japanese patients (median 120 vs 160 cells/mm3).
Interpretation: The eosinophil distribution in Japanese patients seems comparable to that of non-Japanese patients; although within multi-country studies, there was a slightly lower median eosinophil count for Japanese patients compared with non-Japanese patients. These findings suggest that blood eosinophil data from global studies are of relevance in Japan.
Keywords: COPD; Japan; blood eosinophil count; percentage blood eosinophil.
Conflict of interest statement
Disclosure All authors, except NH, are full-time employees of Glaxo SmithKline and hold GlaxoSmithKline shares. NH declares research grants to his department from GlaxoSmithKline, as well as consulting fees from GlaxoSmithKline and other pharmaceutical companies. The authors report no other conflicts of interest in this work.
Figures

References
-
- Pascoe S, Locantore N, Dransfield MT, Barnes NC, Pavord ID. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respir Med. 2015;3(6):435–442. - PubMed
-
- Bafadhel M, Davies L, Calverley PM, Aaron SD, Brightling CE, Pavord ID. Blood eosinophil guided prednisolone therapy for exacerbations of COPD: a further analysis. Eur Respir J. 2014;44(3):789–791. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

