Layer- and Cell Type-Specific Modulation of Excitatory Neuronal Activity in the Neocortex
- PMID: 29440997
- PMCID: PMC5797542
- DOI: 10.3389/fnana.2018.00001
Layer- and Cell Type-Specific Modulation of Excitatory Neuronal Activity in the Neocortex
Abstract
From an anatomical point of view the neocortex is subdivided into up to six layers depending on the cortical area. This subdivision has been described already by Meynert and Brodmann in the late 19/early 20. century and is mainly based on cytoarchitectonic features such as the size and location of the pyramidal cell bodies. Hence, cortical lamination is originally an anatomical concept based on the distribution of excitatory neuron. However, it has become apparent in recent years that apart from the layer-specific differences in morphological features, many functional properties of neurons are also dependent on cortical layer or cell type. Such functional differences include changes in neuronal excitability and synaptic activity by neuromodulatory transmitters. Many of these neuromodulators are released from axonal afferents from subcortical brain regions while others are released intrinsically. In this review we aim to describe layer- and cell-type specific differences in the effects of neuromodulator receptors in excitatory neurons in layers 2-6 of different cortical areas. We will focus on the neuromodulator systems using adenosine, acetylcholine, dopamine, and orexin/hypocretin as examples because these neuromodulator systems show important differences in receptor type and distribution, mode of release and functional mechanisms and effects. We try to summarize how layer- and cell type-specific neuromodulation may affect synaptic signaling in cortical microcircuits.
Keywords: acetylcholine; adenosine; barrel cortex; cortical layers; dopamine; neuromodulation; orexin.
Figures
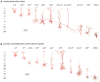
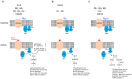

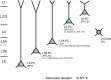
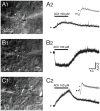


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

