15, 16-Dihydrotanshinone I Inhibits Hemangiomas through Inducing Pro-apoptotic and Anti-angiogenic Mechanisms in Vitro and in Vivo
- PMID: 29441017
- PMCID: PMC5797551
- DOI: 10.3389/fphar.2018.00025
15, 16-Dihydrotanshinone I Inhibits Hemangiomas through Inducing Pro-apoptotic and Anti-angiogenic Mechanisms in Vitro and in Vivo
Abstract
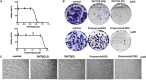
Infantile hemangioma (IH) is a common and benign vascular neoplasms, which has a high incidence in children. Although IH is benign, some patients experience complications such as pain, functional impairment, and permanent disfigurement. Treatment options for IH include corticosteroids, surgery, vincristine, interferon or cyclophosphamide. However, none of these modalities are ideal due to restrictions or potential serious side effects. There is thus a great need to explore novel treatments for IH with less side effects. Angiogenesis, vasculogenesis and tumorigenesis are the main features of IH. Tanshen is mostly used in Chinese traditional medicine to treat hematological abnormalities. Therefore, the aim of our study was to evaluate anti-proliferation and anti-angiogenesis effects on hemangiomas cells by extracted Tanshen compounds compared with propranolol, the first-line treatment for IH currently, both in vitro and in vivo. Cell viability, apoptosis, protein expression and anti-angiogenesis were analyzed by CCK8, Annexin V staining, Western blot and tube formation, respectively. The anti-tumor activity in vivo was evaluated using a mouse xenograft model. Fourteen major compounds extracting from Tanshen were screened for their ability to inhibit hemangiomas cells. Of the 14 compounds investigated, 15,16-Dihydrotanshinone I (DHTS) was the most potent modulator of EOMA cell biology. DHTS could significantly decrease EOMA cells proliferation by inducing cell apoptosis, which is much more efficient than propranolol in vitro. DHTS increased the expression of several apoptosis-related proteins, including caspase9, caspase3, PARP, AIF, BAX, cytochrome c, caspase8 and FADD and significantly inhibited angiogenesis, as indicated by reduced tube formation and diminished expression of vascular endothelial cell growth factor receptor 2 and matrix metalloproteinase 9. In nude mice xenograft experiment, DHTS (10 mg/kg) could significantly inhibit the tumor growth of EOMA cells as well as propranolol (40 mg/kg). Our study showed that DHTS was much more effective than propranolol in inhibiting hemangiomas proliferation and angiogenesis in vitro and in vivo, which could have potential therapeutic applications for treatment of IH.
Keywords: 15,16-dihydrotanshinone I; anti-angiogenesis; apoptosis; infantile hemangiomas; propranolol.
Figures







References
-
- Albiñana V., Villar Gómez de Las Heras K., Serrano-Heras G., Segura T., Perona-Moratalla A. B., Mota-Pérez M. (2015). Propranolol reduces viability and induces apoptosis in hemangioblastoma cells from von Hippel-Lindau patients. Orphanet J. Rare Dis. 10:118. 10.1186/s13023-015-0343-5 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

