Calpain activation and disturbance of autophagy are induced in cortical neurons in vitro by exposure to HA/ β-Ga2O3:Cr3+ nanoparticles
- PMID: 29441243
- PMCID: PMC5807884
- DOI: 10.7717/peerj.4365
Calpain activation and disturbance of autophagy are induced in cortical neurons in vitro by exposure to HA/ β-Ga2O3:Cr3+ nanoparticles
Abstract
The toxicity of engineered nanoparticles remains a concern. The knowledge of biohazards associated with particular nanoparticles is crucial to make this cutting-edge technology more beneficial and safe. Here, we evaluated the toxicity of Ga2O3 nanoparticles (NPs), which are frequently used to enhance the performance of metal catalysts in a variety of catalytic reactions. The potential inflammatory signaling associated with the toxicity of HA/β-Ga2O3:Cr3+ NPs in primary cortical neurons was examined. We observed a dose-dependent decrease in cell viability and an increase in apoptosis in neurons following various concentrations (0, 1, 5, 25, 50, 100 µg/ml) of HA/β-Ga2O3:Cr3+ NPs treatment. Consistently, constitutively active forms of calcineurin (48 kDa) were significantly elevated in cultured primary cortical neurons, which was consistent with calpain activation indicated by the breakdown products of spectrin. Moreover, HA/β-Ga2O3:Cr3+ NPs result in the elevation of LC3-II formation, SQSTM/p62, and Cathepsin B, whereas phosphorylation of CaMKII (Thr286) and Synapsin I (Ser603) were downregulated in the same context. Taken together, these results demonstrate for the first time that calpain activation and a disturbance of autophagy signaling are evoked by exposure to HA/β-Ga2O3:Cr3+ NPs, which may contribute to neuronal injury in vitro.
Keywords: Autophagy; Calpain; HA/β-Ga2O3:Cr3+; Inflammation; Nanoparticles; Neurotoxicity.
Conflict of interest statement
The authors declare there are no competing interests.
Figures


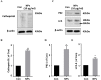
References
-
- Calderon-Garciduenas L, Solt AC, Henriquez-Roldan C, Torres-Jardon R, Nuse B, Herritt L, Villarreal-Calderon R, Osnaya N, Stone I, Garcia R, Brooks DM, Gonzalez-Maciel A, Reynoso-Robles R, Delgado-Chavez R, Reed W. Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid beta-42 and alpha-synuclein in children and young adults. Toxicologic Pathology. 2008;36:289–310. doi: 10.1177/0192623307313011. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

