Spatial structure of disordered proteins dictates conductance and selectivity in nuclear pore complex mimics
- PMID: 29442997
- PMCID: PMC5826291
- DOI: 10.7554/eLife.31510
Spatial structure of disordered proteins dictates conductance and selectivity in nuclear pore complex mimics
Abstract
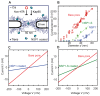
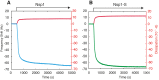
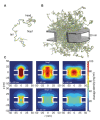
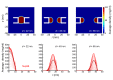
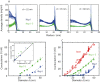
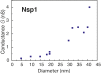
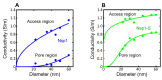
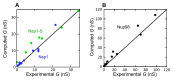
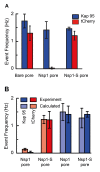
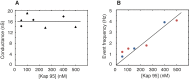
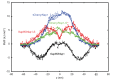
Nuclear pore complexes (NPCs) lined with intrinsically disordered FG-domains act as selective gatekeepers for molecular transport between the nucleus and the cytoplasm in eukaryotic cells. The underlying physical mechanism of the intriguing selectivity is still under debate. Here, we probe the transport of ions and transport receptors through biomimetic NPCs consisting of Nsp1 domains attached to the inner surface of solid-state nanopores. We examine both wildtype FG-domains and hydrophilic SG-mutants. FG-nanopores showed a clear selectivity as transport receptors can translocate across the pore whereas other proteins cannot. SG mutant pores lack such selectivity. To unravel this striking difference, we present coarse-grained molecular dynamics simulations that reveal that FG-pores exhibit a high-density, nonuniform protein distribution, in contrast to a uniform and significantly less-dense protein distribution in the SG-mutant. We conclude that the sequence-dependent density distribution of disordered proteins inside the NPC plays a key role for its conductivity and selective permeability.
Keywords: FG-Nups; Nuclear Pore Complex; biophysics; none; selective barrier; solid-state nanopores; structural biology.
© 2018, Ananth et al.
Conflict of interest statement
AA, AM, SF, AD, RV, Ev, DG, PO, CD No competing interests declared
Figures











Similar articles
-
A designer FG-Nup that reconstitutes the selective transport barrier of the nuclear pore complex.Nat Commun. 2021 Mar 31;12(1):2010. doi: 10.1038/s41467-021-22293-y. Nat Commun. 2021. PMID: 33790297 Free PMC article.
-
Deciphering the intrinsically disordered characteristics of the FG-Nups through the lens of polymer physics.Nucleus. 2024 Dec;15(1):2399247. doi: 10.1080/19491034.2024.2399247. Epub 2024 Sep 16. Nucleus. 2024. PMID: 39282864 Free PMC article. Review.
-
DNA origami scaffold for studying intrinsically disordered proteins of the nuclear pore complex.Nat Commun. 2018 Mar 2;9(1):902. doi: 10.1038/s41467-018-03313-w. Nat Commun. 2018. PMID: 29500415 Free PMC article.
-
The Effect of FG-Nup Phosphorylation on NPC Selectivity: A One-Bead-Per-Amino-Acid Molecular Dynamics Study.Int J Mol Sci. 2019 Jan 30;20(3):596. doi: 10.3390/ijms20030596. Int J Mol Sci. 2019. PMID: 30704069 Free PMC article.
-
Modeling the nucleoporins that form the hairy pores.Biochem Soc Trans. 2020 Aug 28;48(4):1447-1461. doi: 10.1042/BST20190941. Biochem Soc Trans. 2020. PMID: 32794558 Review.
Cited by
-
Theoretical Modeling of Chemical Equilibrium in Weak Polyelectrolyte Layers on Curved Nanosystems.Polymers (Basel). 2020 Oct 5;12(10):2282. doi: 10.3390/polym12102282. Polymers (Basel). 2020. PMID: 33027995 Free PMC article. Review.
-
Molecular basis of C9orf72 poly-PR interference with the β-karyopherin family of nuclear transport receptors.Sci Rep. 2022 Dec 9;12(1):21324. doi: 10.1038/s41598-022-25732-y. Sci Rep. 2022. PMID: 36494425 Free PMC article.
-
A simple thermodynamic description of phase separation of Nup98 FG domains.Nat Commun. 2022 Oct 18;13(1):6172. doi: 10.1038/s41467-022-33697-9. Nat Commun. 2022. PMID: 36257947 Free PMC article.
-
A designer FG-Nup that reconstitutes the selective transport barrier of the nuclear pore complex.Nat Commun. 2021 Mar 31;12(1):2010. doi: 10.1038/s41467-021-22293-y. Nat Commun. 2021. PMID: 33790297 Free PMC article.
-
Deciphering the intrinsically disordered characteristics of the FG-Nups through the lens of polymer physics.Nucleus. 2024 Dec;15(1):2399247. doi: 10.1080/19491034.2024.2399247. Epub 2024 Sep 16. Nucleus. 2024. PMID: 39282864 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

