Environmentally Persistent Free Radicals: Insights on a New Class of Pollutants
- PMID: 29443514
- PMCID: PMC6497067
- DOI: 10.1021/acs.est.7b04439
Environmentally Persistent Free Radicals: Insights on a New Class of Pollutants
Abstract
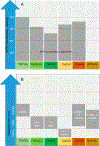
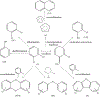
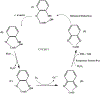
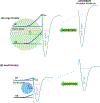
Environmentally persistent free radicals, EPFRs, exist in significant concentration in atmospheric particulate matter (PM). EPFRs are primarily emitted from combustion and thermal processing of organic materials, in which the organic combustion byproducts interact with transition metal-containing particles to form a free radical-particle pollutant. While the existence of persistent free radicals in combustion has been known for over half-a-century, only recently that their presence in environmental matrices and health effects have started significant research, but still in its infancy. Most of the experimental studies conducted to understand the origin and nature of EPFRs have focused primarily on nanoparticles that are supported on a larger micrometer-sized particle that mimics incidental nanoparticles formed during combustion. Less is known on the extent by which EPFRs may form on engineered nanomaterials (ENMs) during combustion or thermal treatment. In this critical and timely review, we summarize important findings on EPFRs and discuss their potential to form on pristine ENMs as a new research direction. ENMs may form EPFRs that may differ in type and concentration compared to nanoparticles that are supported on larger particles. The lack of basic data and fundamental knowledge about the interaction of combustion byproducts with ENMs under high-temperature and oxidative conditions present an unknown environmental and health burden. Studying the extent of ENMs on catalyzing EPFRs is important to address the hazards of atmospheric PM fully from these emerging environmental contaminants.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

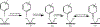
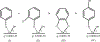
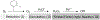
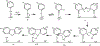
References
-
- Lomnicki S; Truong H; Vejerano E; Dellinger B Copper oxide-based model of persistent free radical formation on combustion-derived particulate matter. Environ. Sci. Technol 2008, 42 (13), 4982–4988. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

