Tissue stiffening coordinates morphogenesis by triggering collective cell migration in vivo
- PMID: 29443958
- PMCID: PMC6013044
- DOI: 10.1038/nature25742
Tissue stiffening coordinates morphogenesis by triggering collective cell migration in vivo
Abstract
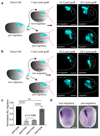
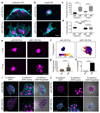
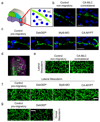
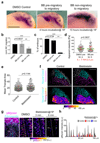
Collective cell migration is essential for morphogenesis, tissue remodelling and cancer invasion. In vivo, groups of cells move in an orchestrated way through tissues. This movement involves mechanical as well as molecular interactions between cells and their environment. While the role of molecular signals in collective cell migration is comparatively well understood, how tissue mechanics influence collective cell migration in vivo remains unknown. Here we investigated the importance of mechanical cues in the collective migration of the Xenopus laevis neural crest cells, an embryonic cell population whose migratory behaviour has been likened to cancer invasion. We found that, during morphogenesis, the head mesoderm underlying the cephalic neural crest stiffens. This stiffening initiates an epithelial-to-mesenchymal transition in neural crest cells and triggers their collective migration. To detect changes in their mechanical environment, neural crest cells use mechanosensation mediated by the integrin-vinculin-talin complex. By performing mechanical and molecular manipulations, we show that mesoderm stiffening is necessary and sufficient to trigger neural crest migration. Finally, we demonstrate that convergent extension of the mesoderm, which starts during gastrulation, leads to increased mesoderm stiffness by increasing the cell density underneath the neural crest. These results show that convergent extension of the mesoderm has a role as a mechanical coordinator of morphogenesis, and reveal a link between two apparently unconnected processes-gastrulation and neural crest migration-via changes in tissue mechanics. Overall, we demonstrate that changes in substrate stiffness can trigger collective cell migration by promoting epithelial-to-mesenchymal transition in vivo. More broadly, our results raise the idea that tissue mechanics combines with molecular effectors to coordinate morphogenesis.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





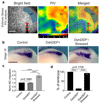
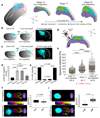

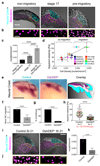
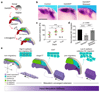
Comment in
-
Cell migration in the cardiovascular system: a force to be reckoned with?Cardiovasc Res. 2018 Sep 1;114(11):e78-e80. doi: 10.1093/cvr/cvy192. Cardiovasc Res. 2018. PMID: 30428021 No abstract available.
References
-
- Roca-Cusachs P, Sunyer R, Trepat X. Mechanical guidance of cell migration: lessons from chemotaxis. Current Opinion in Cell Biology. 2013;25:543–549. - PubMed
-
- Mayor R, Etienne-Manneville S. The front and rear of collective cell migration. Nature Reviews Molecular Cell Biology. 2016;17:97–109. - PubMed
-
- Gilmour D, Rembold M, Leptin M. From morphogen to morphogenesis and back. Nature. 2017;541:311–320. - PubMed
-
- Nieto M, Huang R, Jackson R, Thiery J. EMT: 2016. Cell. 2016;166:21–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

