Promising approach to reducing Malaria transmission by ivermectin: Sporontocidal effect against Plasmodium vivax in the South American vectors Anopheles aquasalis and Anopheles darlingi
- PMID: 29444080
- PMCID: PMC5828505
- DOI: 10.1371/journal.pntd.0006221
Promising approach to reducing Malaria transmission by ivermectin: Sporontocidal effect against Plasmodium vivax in the South American vectors Anopheles aquasalis and Anopheles darlingi
Abstract
Background: The mosquito resistance to the insecticides threatens malaria control efforts, potentially becoming a major public health issue. Alternative methods like ivermectin (IVM) administration to humans has been suggested as a possible vector control to reduce Plasmodium transmission. Anopheles aquasalis and Anopheles darlingi are competent vectors for Plasmodium vivax, and they have been responsible for various malaria outbreaks in the coast of Brazil and the Amazon Region of South America.
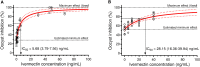
Methods: To determine the IVM susceptibility against P. vivax in An. aquasalis and An. darlingi, ivermectin were mixed in P. vivax infected blood: (1) Powdered IVM at four concentrations (0, 5, 10, 20 or 40 ng/mL). (2) Plasma (0 hours, 4 hours, 1 day, 5, 10 and 14 days) was collected from healthy volunteers after to administer a single oral dose of IVM (200 μg/kg) (3) Mosquitoes infected with P. vivax and after 4 days was provided with IVM plasma collected 4 hours post-treatment (4) P. vivax-infected patients were treated with various combinations of IVM, chloroquine, and primaquine and plasma or whole blood was collected at 4 hours. Seven days after the infective blood meal, mosquitoes were dissected to evaluate oocyst presence. Additionally, the ex vivo effects of IVM against asexual blood-stage P. vivax was evaluated.
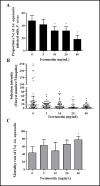
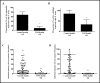
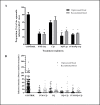
Results: IVM significantly reduced the prevalence of An. aquasalis that developed oocysts in 10 to 40 ng/mL pIVM concentrations and plasma 4 hours, 1 day and 5 days. In An. darlingi to 4 hours and 1 day. The An. aquasalis mortality was expressively increased in pIVM (40ng/mL) and plasma 4 hours, 1, 5 10 and 14 days post-intake drug and in An. darlingi only to 4 hours and 1 day. The double fed meal with mIVM by the mosquitoes has a considerable impact on the proportion of infected mosquitoes for 7 days post-feeding. The oocyst infection prevalence and intensity were notably reduced when mosquitoes ingested blood from P. vivax patients that ingested IVM+CQ, PQ+CQ and IVM+PQ+CQ. P. vivax asexual development was considerably inhibited by mIVM at four-fold dilutions.
Conclusion: In conclusion, whole blood spiked with IVM reduced the infection rate of P. vivax in An. aquasalis and An. darlingi, and increased the mortality of mosquitoes. Plasma from healthy volunteers after IVM administration affect asexual P. vivax development. These findings support that ivermectin may be used to decrease P. vivax transmission.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- WHO. Implications of insecticide resistance for malaria vector control. World Health Organization; http://wwwwhoint/malaria/news/2016/iir-malaria-vectorcontrol-evaluation-.... 2016.
-
- Oliveira-Ferreira J, Lacerda MV, Brasil P, Ladislau JL, Tauil PL, Daniel-Ribeiro CT. Malaria in Brazil: an overview. Malaria journal. 2010;9:115 doi: 10.1186/1475-2875-9-115 - DOI - PMC - PubMed
-
- Recht J, Siqueira AM, Monteiro WM, Herrera SM, Herrera S, Lacerda MVG. Malaria in Brazil, Colombia, Peru and Venezuela: current challenges in malaria control and elimination. Malaria journal. 2017;16(1):273 doi: 10.1186/s12936-017-1925-6 - DOI - PMC - PubMed
-
- Abdallah TM, Ali AA, Bakri M, Gasim GI, Musa IR, Adam I. Efficacy of artemether-lumefantrine as a treatment for uncomplicated Plasmodium vivax malaria in eastern Sudan. Malaria journal. 2012;11:404 doi: 10.1186/1475-2875-11-404 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

