GLP-1 Receptor Expression Within the Human Heart
- PMID: 29444223
- PMCID: PMC5939638
- DOI: 10.1210/en.2018-00004
GLP-1 Receptor Expression Within the Human Heart
Abstract
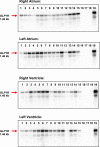
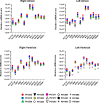
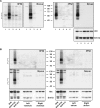
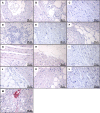
Glucagonlike peptide-1 receptor (GLP-1R) agonists, which are used to treat type 2 diabetes and obesity, reduce the rates of myocardial infarction and cardiovascular death. GLP-1R has been localized to the human sinoatrial node; however, its expression in ventricular tissue remains uncertain. Here we studied GLP-1R expression in the human heart using GLP-1R-directed antisera, quantitative polymerase chain reaction (PCR), reverse transcription PCR to detect full-length messenger RNA (mRNA) transcripts, and in situ hybridization (ISH). GLP1R mRNA transcripts, encompassing the entire open reading frame, were detected in all four cardiac chambers from 15 hearts at levels approximating those detected in human pancreas. In contrast, cardiac GLP2R expression was relatively lower, and cardiac GCGR expression was sporadic and not detected in the left ventricle. GLP1R mRNA transcripts were not detected in RNA from human cardiac fibroblasts, coronary artery endothelial, or vascular smooth muscle cells. Human Brunner glands and pancreatic islets exhibited GLP-1R immunopositivity and abundant expression of GLP1R mRNA transcripts by ISH. GLP1R transcripts were also detected by ISH in human cardiac sinoatrial node tissue. However, definitive cellular localization of GLP1R mRNA transcripts or immunoreactive GLP-1R protein within human cardiomyocytes or cardiac blood vessels remained elusive. Moreover, validated GLP-1R antisera lacked sufficient sensitivity to detect expression of the endogenous islet or cardiac GLP-1R by Western blotting. Hence, although human cardiac ventricles express the GLP1R, the identity of one or more ventricular cell type(s) that express a translated GLP1R protein requires further clarification with highly sensitive methods of detection.
Figures





Comment in
-
The Cardiac Glucagonlike Peptide-1 Receptor: Whither Art Thou?Endocrinology. 2018 Apr 1;159(4):1842-1843. doi: 10.1210/en.2018-00186. Endocrinology. 2018. PMID: 29509920 No abstract available.
-
Letter to the Editor: "GLP-1 Receptor Expression Within the Human Heart".Endocrinology. 2018 May 1;159(5):1964-1965. doi: 10.1210/en.2018-00196. Endocrinology. 2018. PMID: 29554319 No abstract available.
References
-
- Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132(6):2131–2157. - PubMed
-
- Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17(6):819–837. - PubMed
-
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368(9548):1696–1705. - PubMed
-
- Astrup A, Rössner S, Van Gaal L, Rissanen A, Niskanen L, Al Hakim M, Madsen J, Rasmussen MF, Lean ME; NN8022-1807 Study Group . Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet. 2009;374(9701):1606–1616. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

