Ice algal bloom development on the surface of the Greenland Ice Sheet
- PMID: 29444265
- PMCID: PMC6018781
- DOI: 10.1093/femsec/fiy025
Ice algal bloom development on the surface of the Greenland Ice Sheet
Abstract
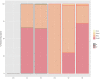
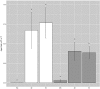
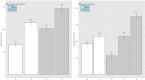
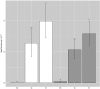
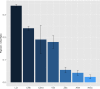
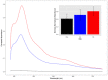
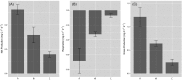
It is fundamental to understand the development of Zygnematophycean (Streptophyte) micro-algal blooms within Greenland Ice Sheet (GrIS) supraglacial environments, given their potential to significantly impact both physical (melt) and chemical (carbon and nutrient cycling) surface characteristics. Here, we report on a space-for-time assessment of a GrIS ice algal bloom, achieved by sampling an ∼85 km transect spanning the south-western GrIS bare ice zone during the 2016 ablation season. Cell abundances ranged from 0 to 1.6 × 104 cells ml-1, with algal biomass demonstrated to increase in surface ice with time since snow line retreat (R2 = 0.73, P < 0.05). A suite of light harvesting and photo-protective pigments were quantified across transects (chlorophylls, carotenoids and phenols) and shown to increase in concert with algal biomass. Ice algal communities drove net autotrophy of surface ice, with maximal rates of net production averaging 0.52 ± 0.04 mg C l-1 d-1, and a total accumulation of 1.306 Gg C (15.82 ± 8.14 kg C km-2) predicted for the 2016 ablation season across an 8.24 × 104 km2 region of the GrIS. By advancing our understanding of ice algal bloom development, this study marks an important step toward projecting bloom occurrence and impacts into the future.
Figures




References
-
- Anesio AM, Hodson AJ, Andreas F et al. . High microbial activity on glaciers: importance to the global carbon cycle. Glob Chang Biol. 2009;15:955–60.
-
- Box JE, Fettweis X, Stroeve JC et al. . The cryosphere Greenland ice sheet albedo feedback: thermodynamics and atmospheric drivers. 2012;6:821–39.
-
- Cameron KA, Stibal M, Hawkings JR et al. . Meltwater export of prokaryotic cells from the Greenland ice sheet. Environ Microbiol. 2017;19:524–34. - PubMed
-
- Chandler DM, Alcock JD, Wadham JL, et al. . Seasonal changes of ice surface characteristics and productivity in the ablation zone of the Greenland Ice Sheet. The Cryosphere. 2015;9:487–504.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

