Secukinumab demonstrates high sustained efficacy and a favourable safety profile in patients with moderate-to-severe psoriasis through 5 years of treatment (SCULPTURE Extension Study)
- PMID: 29444376
- PMCID: PMC6175198
- DOI: 10.1111/jdv.14878
Secukinumab demonstrates high sustained efficacy and a favourable safety profile in patients with moderate-to-severe psoriasis through 5 years of treatment (SCULPTURE Extension Study)
Abstract
Background: Secukinumab, a fully human monoclonal antibody that selectively neutralizes IL-17A, has been shown to have significant efficacy and a favourable safety profile in the treatment of moderate-to-severe psoriasis and psoriatic arthritis.
Objective: To assess the efficacy and safety of secukinumab through 5 years of treatment in moderate-to-severe psoriasis.
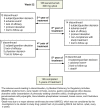
Methods: In the core SCULPTURE study, Psoriasis Area and Severity Index (PASI) 75 responders at Week 12 continued receiving subcutaneous secukinumab until Year 1. Thereafter, patients entered the extension phase and continued treatment as per the core trial. Treatment was double-blinded until the end of Year 3 and open-label from Year 4. Here, we focus on the 300 mg fixed-interval (every 4 weeks) treatment, the recommended per label dose. Efficacy data are primarily reported as observed, but multiple imputation (MI) and last observation carried forward (LOCF) techniques were also undertaken as supportive analyses.
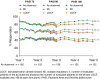
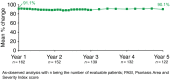
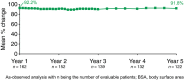
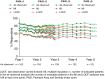
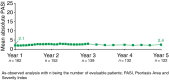
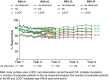
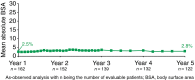
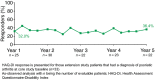
Results: At Year 1, 168 patients entered the extension study and at the end of Year 5, 126 patients completed 300 mg (every 4 weeks) treatment. PASI 75/90/100 responses at Year 1 (88.9%, 68.5% and 43.8%, respectively) were sustained to Year 5 (88.5%, 66.4% and 41%). PASI responses were consistent regardless of the analysis undertaken (as observed, MI, or LOCF). The average improvement in mean PASI was approximately 90% through 5 years compared with core study baseline. DLQI (dermatology life quality index) 0/1 response also sustained through 5 years (72.7% at Year 1 and 65.5% at Year 5). The safety profile of secukinumab remained favourable, with no cumulative or unexpected safety concerns identified.
Conclusion: Secukinumab 300 mg treatment delivered high and sustained levels of skin clearance and improved quality of life through 5 years in patients with moderate-to-severe psoriasis. Favourable safety established in the secukinumab phase 2/3 programme was maintained through 5 years.
© 2018 The Authors. Journal of the European Academy of Dermatology and Venereology published by John Wiley & Sons Ltd on behalf of European Academy of Dermatology and Venereology.
Figures

References
-
- Mease PJ, McInnes IB, Kirkham B et al Secukinumab inhibition of interleukin‐17A in patients with psoriatic arthritis. N Engl J Med 2015; 373: 1329–1339. - PubMed
-
- Langley RG, Elewski BE, Lebwohl M et al Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med 2014; 371: 326–338. - PubMed
-
- Blauvelt A, Reich K, Tsai TF et al Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate‐to‐severe plaque psoriasis up to 1 year: results from the CLEAR study. J Am Acad Dermatol 2017; 76: 60–69. - PubMed
-
- Thaci D, Blauvelt A, Reich K et al Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol 2015; 73: 400–409. - PubMed
-
- Hueber W, Patel DD, Dryja T et al Effects of AIN457, a fully human antibody to interleukin‐17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med 2010; 2: 52ra72. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

