MicroRNA-223 Suppresses the Canonical NF-κB Pathway in Basal Keratinocytes to Dampen Neutrophilic Inflammation
- PMID: 29444433
- PMCID: PMC5839657
- DOI: 10.1016/j.celrep.2018.01.058
MicroRNA-223 Suppresses the Canonical NF-κB Pathway in Basal Keratinocytes to Dampen Neutrophilic Inflammation
Abstract
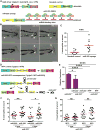
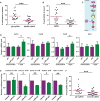
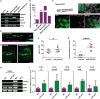
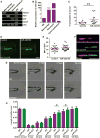
MicroRNA-223 is known as a myeloid-enriched anti-inflammatory microRNA that is dysregulated in numerous inflammatory conditions. Here, we report that neutrophilic inflammation (wound response) is augmented in miR-223-deficient zebrafish, due primarily to elevated activation of the canonical nuclear factor κB (NF-κB) pathway. NF-κB over-activation is restricted to the basal layer of the surface epithelium, although miR-223 is detected throughout the epithelium and in phagocytes. Not only phagocytes but also epithelial cells are involved in miR-223-mediated regulation of neutrophils' wound response and NF-κB activation. Cul1a/b, Traf6, and Tab1 are identified as direct targets of miR-223, and their levels rise in injured epithelium lacking miR-223. In addition, miR-223 is expressed in cultured human bronchial epithelial cells, where it also downregulates NF-κB signaling. Together, this direct connection between miR-223 and the canonical NF-κB pathway provides a mechanistic understanding of the multifaceted role of miR-223 and highlights the relevance of epithelial cells in dampening neutrophil activation.
Keywords: NF-κB; epithelium; inflammation; miR-223; microRNA; neutrophils; zebrafish.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Aggad D, Stein C, Sieger D, Mazel M, Boudinot P, Herbomel P, Levraud JP, Lutfalla G, Leptin M. In vivo analysis of Ifn-γ1 and Ifn-γ2 signaling in zebrafish. J. Immunol. 2010;185:6774–6782. - PubMed
-
- Bauernfeind F, Rieger A, Schildberg FA, Knolle PA, Schmid-Burgk JL, Hornung V. NLRP3 inflammasome activity is negatively controlled by miR-223. J. Immunol. 2012;189:4175–4181. - PubMed
-
- Borregaard N. Neutrophils, from marrow to microbes. Immunity. 2010;33:657–670. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

