Measuring Integrin Conformational Change on the Cell Surface with Super-Resolution Microscopy
- PMID: 29444440
- PMCID: PMC5851489
- DOI: 10.1016/j.celrep.2018.01.062
Measuring Integrin Conformational Change on the Cell Surface with Super-Resolution Microscopy
Abstract
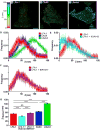
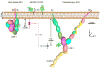
We use super-resolution interferometric photoactivation and localization microscopy (iPALM) and a constrained photoactivatable fluorescent protein integrin fusion to measure the displacement of the head of integrin lymphocyte function-associated 1 (LFA-1) resulting from integrin conformational change on the cell surface. We demonstrate that the distance of the LFA-1 head increases substantially between basal and ligand-engaged conformations, which can only be explained at the molecular level by integrin extension. We further demonstrate that one class of integrin antagonist maintains the bent conformation, while another antagonist class induces extension. Our molecular scale measurements on cell-surface LFA-1 are in excellent agreement with distances derived from crystallographic and electron microscopy structures of bent and extended integrins. Our distance measurements are also in excellent agreement with a previous model of LFA-1 bound to ICAM-1 derived from the orientation of LFA-1 on the cell surface measured using fluorescence polarization microscopy.
Keywords: adhesion receptors; cell adhesion; cell migration; cell-surface receptors; iPALM; immunology; integrins; lymphocyte; protein conformation; super-resolution microscopy.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Betzig E, Patterson GH, Sougrat R, Lindwasser OW, Olenych S, Bonifacino JS, Davidson MW, Lippincott-Schwartz J, Hess HF. Imaging intracellular fluorescent proteins at nanometer resolution. Science. 2006;313:1642–1645. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

